Analysis on the Research Progress of Biosimilar Drugs
Biosimilar drugs: These are therapeutic biological products that have similarity in quality, safety, and efficacy with a previously registered reference drug.
The evaluation of biosimilar drugs mainly focuses on the similarity in quality, effectiveness, and safety. The non-clinical stage mainly evaluates the degree of similarity or difference in pharmacological toxicological characteristics, mainly involving pharmacodynamics (PD), pharmacokinetics (PK), and toxicology research projects, providing a reference for the product's clinical trial phase, subsequent clinical trial design, and evaluation requirements. Meanwhile, the extrapolation of indications for biosimilars, changes in production processes or sites, and other processes also need to be supported by the analysis and evaluation of the product's non-clinical characteristics, in order to obtain the basis for related registration applications.
Biosimilar Competitive Landscape
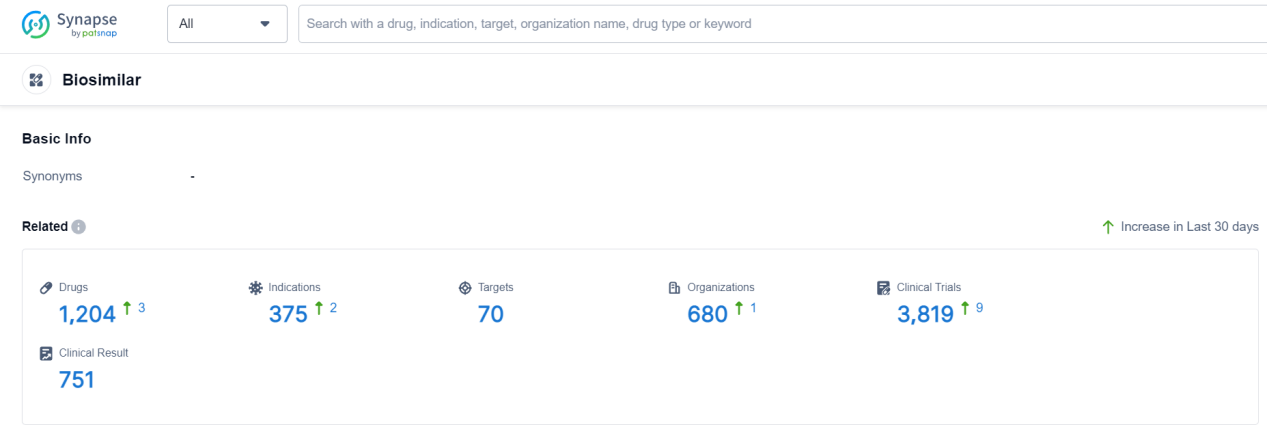
According to Patsnap Synapse, as of 19 Sep 2023, there are a total of 1204 Biosimilar drugs worldwide, from 680 organizations, covering 70 targets, 375 indications, and conducting 3819 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this drug type.
Approved Biosimilar Medicinal: Ozoralizumab
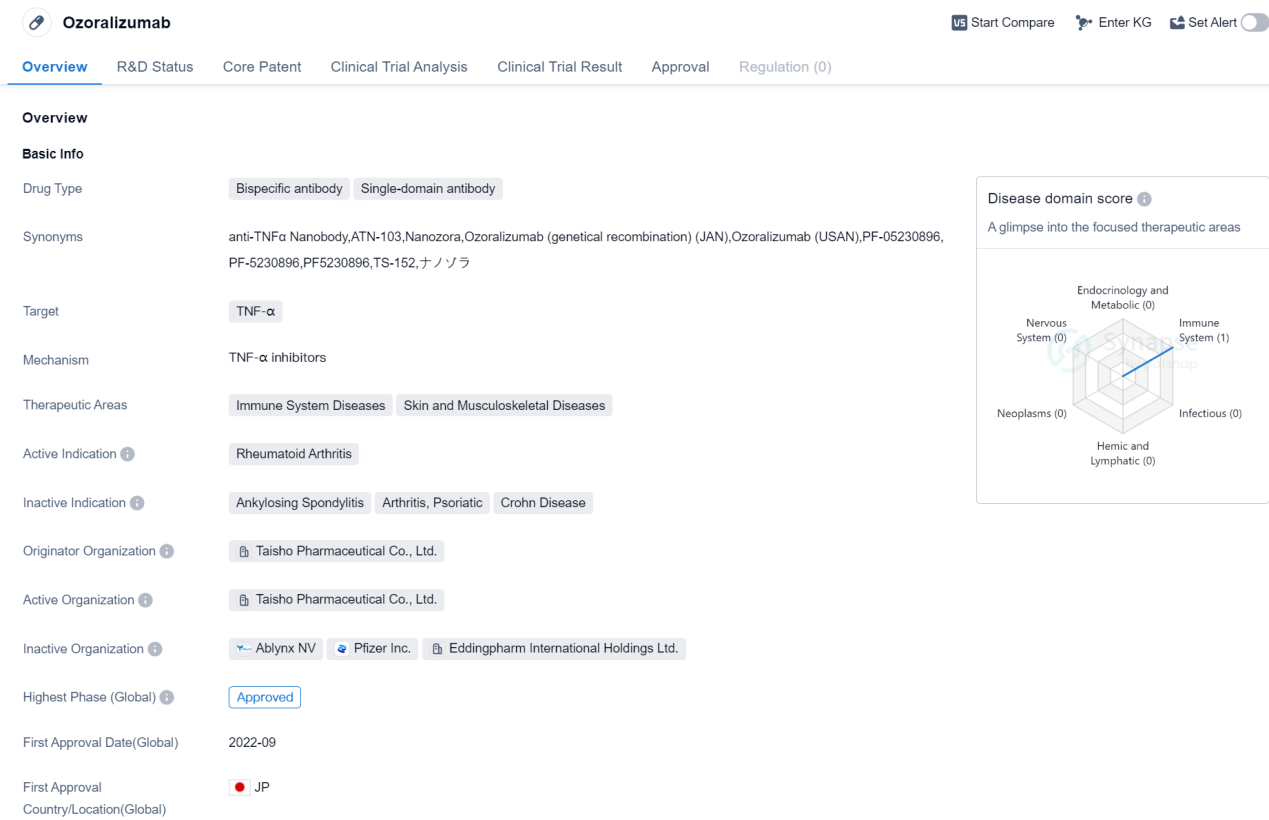
Ozoralizumab is a bispecific antibody and single-domain antibody drug that targets TNF-α. It is primarily used for the treatment of Rheumatoid Arthritis, a chronic autoimmune disease that affects the joints. The drug is developed by Taisho Pharmaceutical Co., Ltd., a pharmaceutical company based in Japan.
Ozoralizumab falls under the therapeutic areas of Immune System Diseases and Skin and Musculoskeletal Diseases. These areas encompass a wide range of conditions related to the immune system and disorders affecting the skin, muscles, and bones.
As of now, Ozoralizumab has reached the highest phase of approval globally, indicating that it has successfully completed clinical trials and has been deemed safe and effective for use. However, its highest phase in China is still pending, suggesting that it is yet to receive approval in the Chinese market.
The drug received its first approval in Japan in September 2022, making it available for use in the country. The specific country or location of the first approval is not mentioned in the provided information.
Ozoralizumab's mechanism of action involves targeting TNF-α, a pro-inflammatory cytokine involved in various immune responses. By inhibiting TNF-α, the drug aims to reduce inflammation and alleviate symptoms associated with Rheumatoid Arthritis.
It is important to note that the provided information is objective and does not include any fictional data or subjective interpretations. The summary provides a concise overview of the drug, its therapeutic areas, indication, originator organization, highest phases of approval, and first approval details.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Approved Biosimilar Medicinal: Golimumab
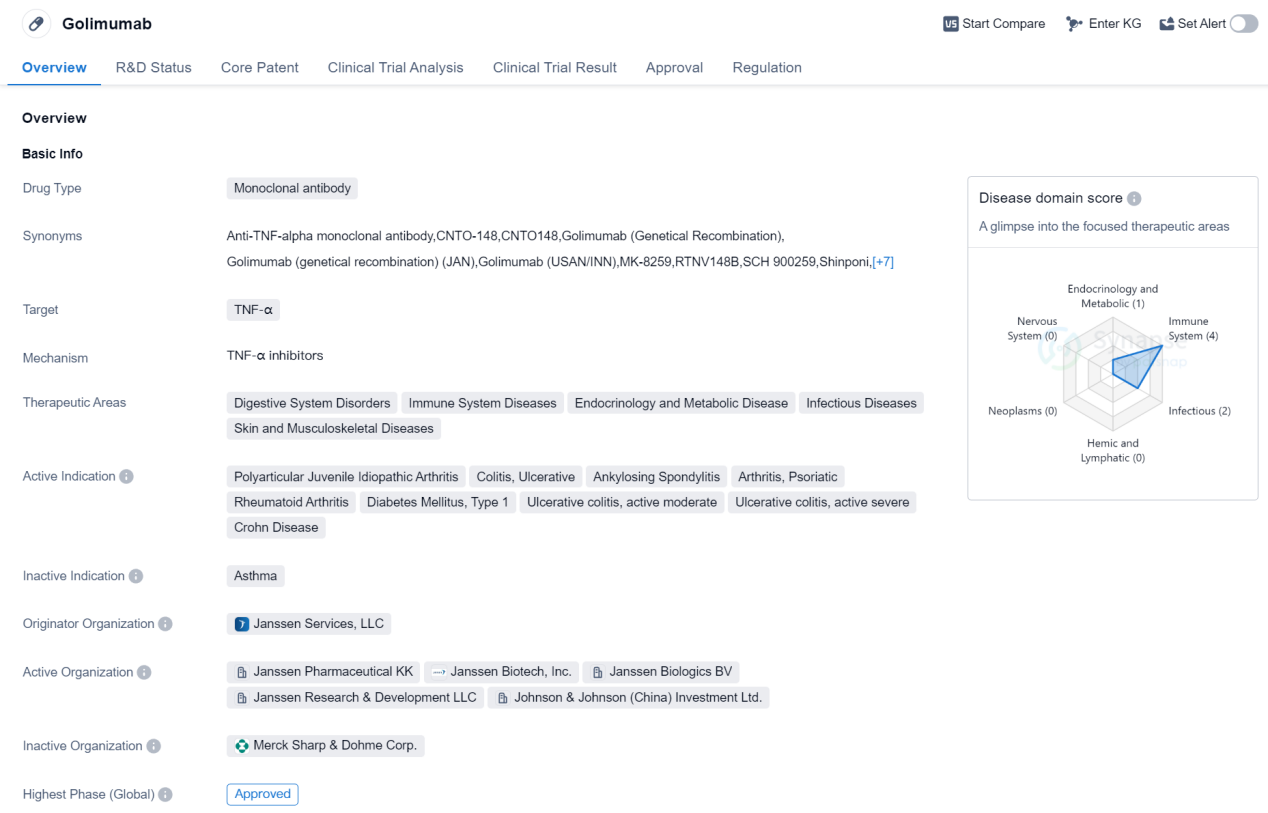
Golimumab is a monoclonal antibody drug that targets TNF-α, making it a valuable treatment option for various therapeutic areas. These areas include digestive system disorders, immune system diseases, endocrinology and metabolic diseases, infectious diseases, and skin and musculoskeletal diseases. The drug has been approved for multiple indications, including polyarticular juvenile idiopathic arthritis, colitis, ulcerative, ankylosing spondylitis, arthritis, psoriatic, rheumatoid arthritis, diabetes mellitus type 1, ulcerative colitis (active moderate and severe), and Crohn's disease.
The originator organization of Golimumab is Janssen Services, LLC. The drug has achieved the highest phase of approval globally. It received its first approval in the United States in April 2009. Golimumab is regulated as an orphan drug, indicating its potential to treat rare diseases or conditions.
As a monoclonal antibody, Golimumab works by targeting TNF-α, a cytokine involved in inflammation. By inhibiting TNF-α, Golimumab helps reduce inflammation and alleviate symptoms associated with the aforementioned therapeutic areas. This mechanism of action has proven effective in managing various diseases, particularly those involving the immune system and chronic inflammation.
The approval of Golimumab in multiple indications highlights its versatility and potential to address a wide range of medical conditions. Its success in the market can be attributed to its ability to target TNF-α, a key player in many inflammatory processes. The drug's approval as an orphan drug further emphasizes its potential to address unmet medical needs and provide treatment options for rare diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Overall, Golimumab's approval and success in the pharmaceutical industry demonstrate its significance in the field of biomedicine. Its ability to target TNF-α and its broad therapeutic areas make it a valuable asset in the treatment of various diseases. As the drug continues to be utilized and researched, it has the potential to further contribute to advancements in the pharmaceutical industry and improve patient outcomes.