Analysis on the Research Progress of HIV-1 Protease Inhibitors
HIV-1 protease is an enzyme that plays a crucial role in the replication of the human immunodeficiency virus (HIV-1) within the human body. It is responsible for cleaving long viral polyproteins into smaller functional proteins, which are essential for the assembly of new infectious viral particles. By targeting and inhibiting the activity of HIV-1 protease, antiretroviral drugs can effectively disrupt the viral replication cycle, preventing the production of mature and infectious HIV particles. This inhibition is vital in managing HIV infection and reducing the viral load in patients, ultimately improving their immune function and slowing down the progression of the disease.
HIV-1 protease Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 18 Sep 2023, there are a total of 177 HIV-1 protease drugs worldwide, from 108 organizations, covering 14 indications, and conducting 925 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.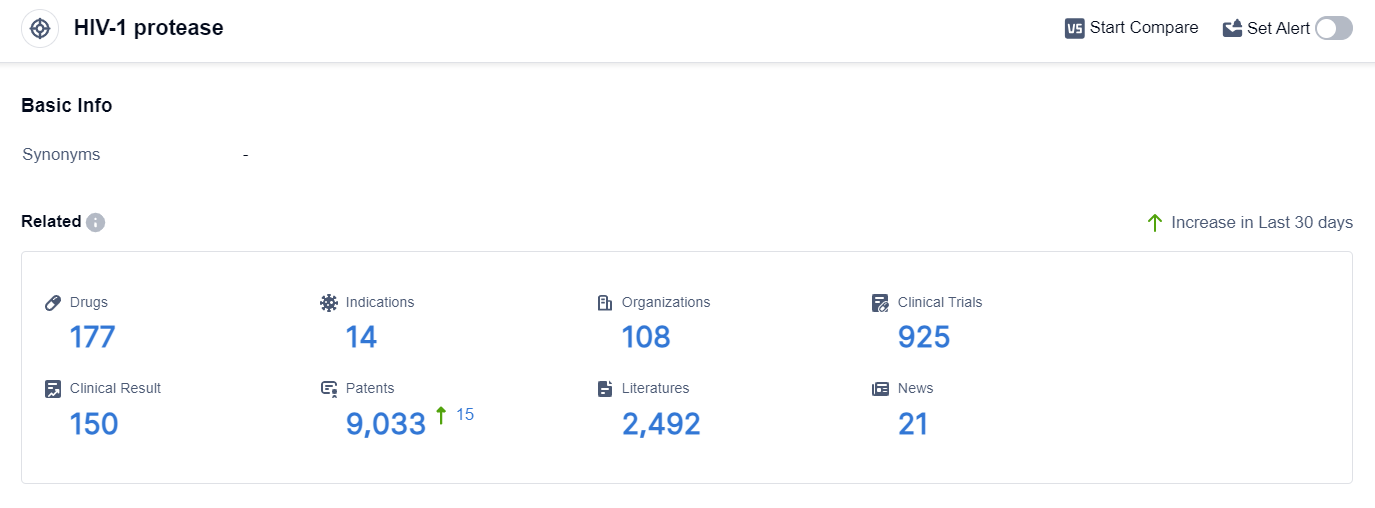
Based on the analysis of the provided data, the current competitive landscape of the target HIV-1 protease is characterized by the involvement of multiple companies, with Bristol Myers Squibb Co., Roche Holding AG, and Merck & Co., Inc. leading in terms of approved drugs.
The primary indications for these drugs are HIV infections and HIV-1 infection, with some drugs also approved for COVID-19. Small molecule drugs dominate the drug types, with synthetic peptides also being developed. Japan, China, and the European Union are the leading countries/locations in terms of drug development under this target. China, in particular, has shown significant progress with 6 approved drugs.
Overall, the target HIV-1 protease presents a competitive landscape with ongoing research and development efforts, indicating potential future advancements in the field.
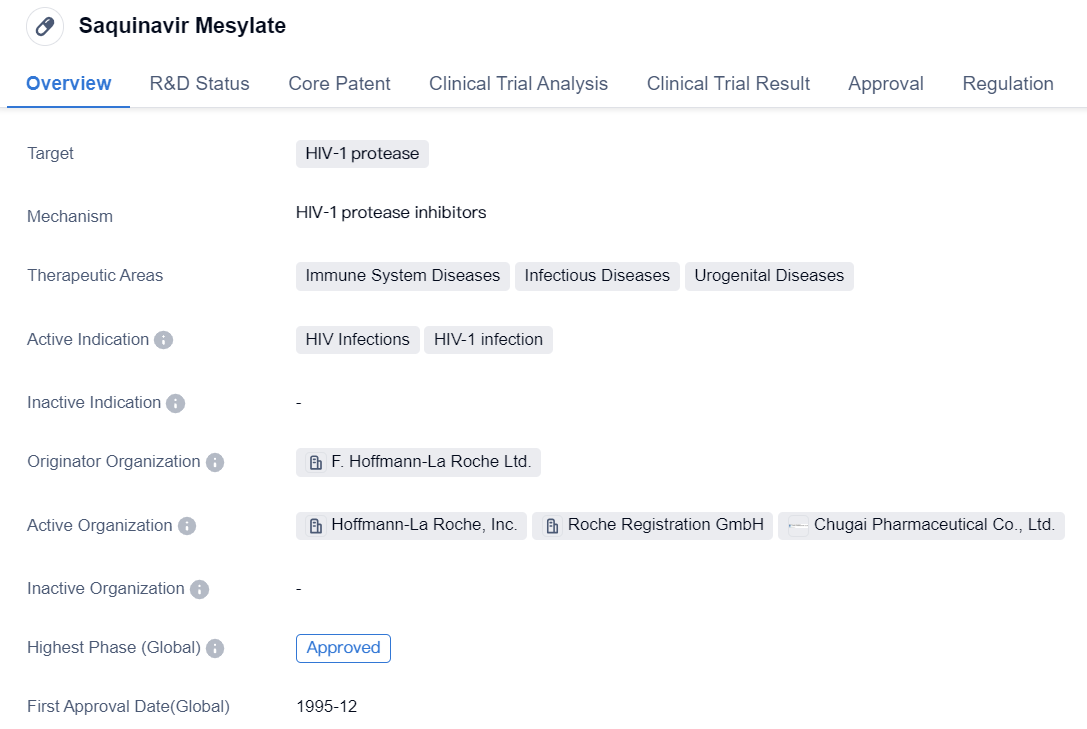
First-generation HIV-1 protease inhibitors: Saquinavir Mesylate
Saquinavir Mesylate is a small molecule drug that is primarily used for the treatment of HIV-1 infections. It specifically targets the HIV-1 protease, which plays a crucial role in the replication of the virus. By inhibiting this enzyme, Saquinavir Mesylate helps to prevent the virus from multiplying and spreading throughout the body.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The drug has been approved for use in various therapeutic areas, including immune system diseases, infectious diseases, and urogenital diseases. This indicates its potential effectiveness in treating a range of conditions related to the immune system and infections, particularly those caused by HIV-1.
Saquinavir Mesylate was first approved for use in the United States in December 1995, making it one of the early drugs in the fight against HIV/AIDS. It was developed by F. Hoffmann-La Roche Ltd., a renowned pharmaceutical company known for its contributions to the field of biomedicine.
The drug has received accelerated approval and orphan drug status, which highlights its potential to address unmet medical needs and rare diseases. Accelerated approval is a regulatory pathway that allows for the expedited approval of drugs that demonstrate promising early results, while orphan drug status provides incentives for the development of drugs targeting rare diseases.
Saquinavir Mesylate has successfully completed the highest phase of clinical trials and has been approved for use globally, including in China. This indicates that it has undergone rigorous testing and has been deemed safe and effective for its intended use.
In summary, Saquinavir Mesylate is a small molecule drug developed by F. Hoffmann-La Roche Ltd. It targets the HIV-1 protease and has been approved for the treatment of HIV-1 infections. It has shown potential in various therapeutic areas, including immune system diseases, infectious diseases, and urogenital diseases. The drug received accelerated approval and orphan drug status, and it was first approved in the United States in 1995. Saquinavir Mesylate has completed the highest phase of clinical trials and is approved for use globally.
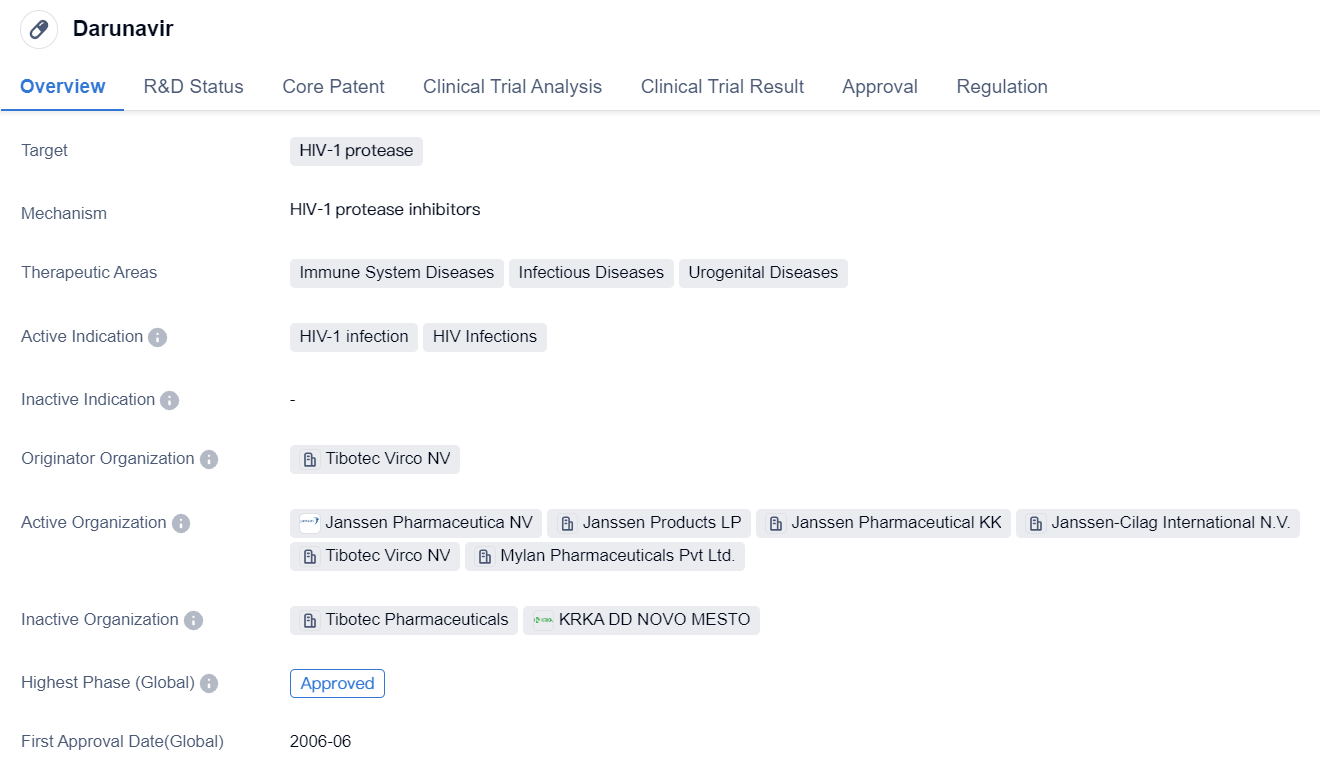
Second-Generation HIV-1 Protease Inhibitors: Darunavir
Darunavir is a small molecule drug that is primarily used for the treatment of HIV-1 infection. It specifically targets the HIV-1 protease, which plays a crucial role in the replication of the virus. By inhibiting this enzyme, darunavir helps to prevent the virus from multiplying and spreading throughout the body.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The drug has been approved for use in various therapeutic areas, including immune system diseases, infectious diseases, and urogenital diseases. Its active indications are HIV-1 infection and HIV infections, indicating its effectiveness in managing these conditions.
Darunavir was developed by Tibotec Virco NV, an originator organization specializing in the field of biomedicine. It has undergone rigorous testing and clinical trials, resulting in its approval for use in multiple countries.
The highest phase of development for darunavir is approved, indicating that it has successfully completed all necessary stages of testing and has been deemed safe and effective for use in patients. This approval applies both globally and in China, suggesting that the drug has met the regulatory requirements of these regions.
Darunavir received its first approval in the United States in June 2006. This signifies that it was first recognized and authorized for use in this country. The drug has since gained approval in other countries as well.
In terms of regulation, darunavir has been granted accelerated approval and has also been designated as an orphan drug. Accelerated approval is a regulatory pathway that allows for the expedited approval of drugs that address unmet medical needs. Orphan drug status is granted to medications that are intended to treat rare diseases or conditions.
In summary, darunavir is a small molecule drug developed by Tibotec Virco NV for the treatment of HIV-1 infection. It targets the HIV-1 protease and has been approved for use in various therapeutic areas. The drug has undergone extensive testing and received its first approval in the United States in 2006. It has since been approved globally and in China, with regulatory designations of accelerated approval and orphan drug status.