Analysis on the Research Progress of GnRHR antagonists
GnRH, also known as Gonadotropin-Releasing Hormone, is a decapeptide hormone secreted by the arcuate nucleus neurons in the hypothalamus. Through binding with the GnRH receptor (GnRHR) on the surface of pituitary gonadotrope cell membrane, it forms a ligand-receptor complex that enters the cell, thus stimulating the pituitary to periodically secrete gonadotropins LH (Luteinizing Hormone) and FSH (Follicle-Stimulating Hormone). LH and FSH act on the gonads to promote the secretion of sex hormones, which in turn affect the development of mammary glands, adrenal cortex, thyroid, and bone growth. Due to the wide-ranging effects of sex hormones, many diseases rely on these hormones for their development, such as endometriosis, uterine fibroids, breast cancer, and prostate cancer.
GnRHR Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 18 Sep 2023, there are a total of 81 GnRHR drugs worldwide, from 128 organizations, covering 53 indications, and conducting 1343 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.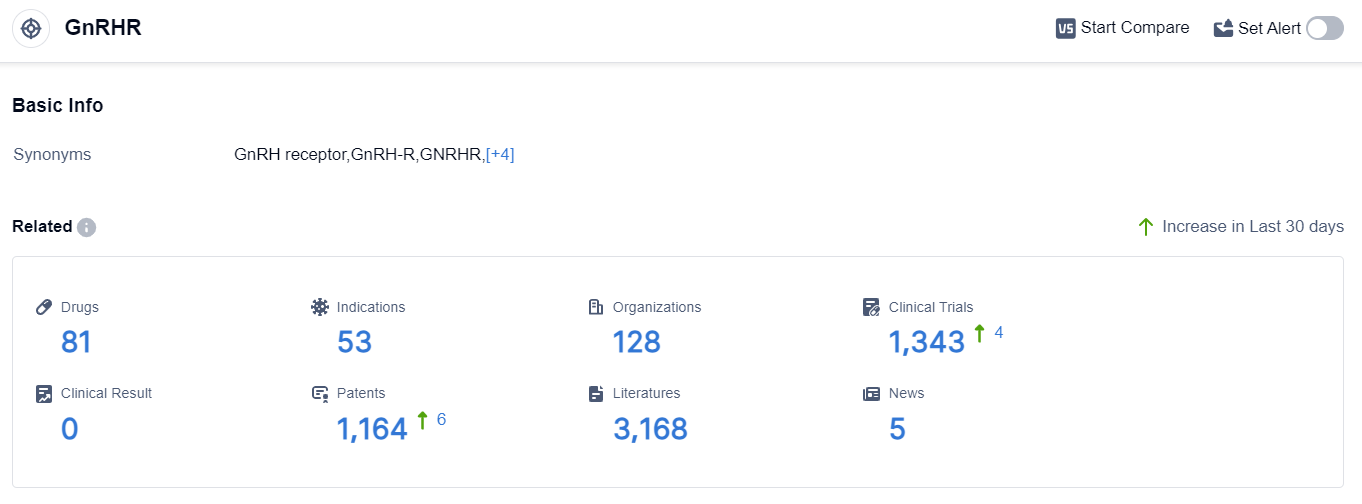
The analysis of the target GnRHR reveals a competitive landscape with multiple companies actively involved in the research and development of drugs. AbbVie, Inc., Sumitomo Chemical Co., Ltd., ObsEva SA, Ipsen SA, and Shanghai Livzon Pharmaceutical Co. Ltd. are the companies growing fastest under the current target.
The indications for the approved drugs targeting GnRHR cover a wide range of conditions related to the reproductive system and hormone regulation. Small molecule drugs and synthetic peptides are the drug types progressing most rapidly, indicating intense competition around the innovative drugs.
The United States, European Union, Japan, China, and Norway are the countries/locations developing fastest under the current target, with China showing progress in the development of drugs for GnRHR. Overall, the target GnRHR presents a competitive landscape with diverse companies, indications, drug types, and countries/locations involved in research and development.
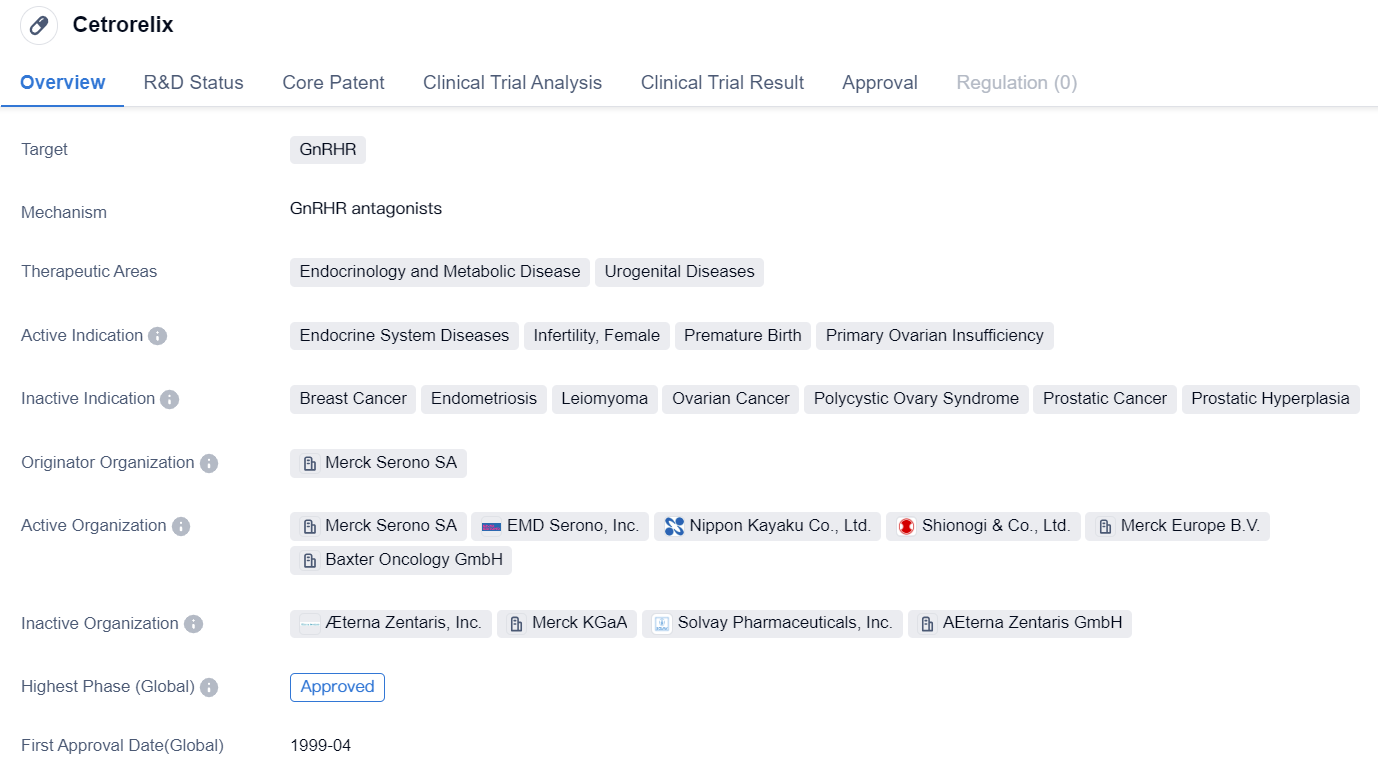
Key Drug: Cetrorelix
Cetrorelix is a synthetic peptide drug that targets the GnRHR. It falls under the therapeutic areas of endocrinology and metabolic disease, as well as urogenital diseases. The drug is indicated for the treatment of various conditions related to the endocrine system, infertility in females, premature birth, and primary ovarian insufficiency.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Cetrorelix was developed by Merck Serono SA, a prominent organization in the pharmaceutical industry. It has successfully completed the highest phase of clinical trials and has received approval both globally and in China. The drug was first approved in the European Union in April 1999, marking its initial entry into the market.
As a synthetic peptide, Cetrorelix is designed to mimic the actions of natural peptides in the body. By targeting the GnRHR, it modulates the release of gonadotropins, which are hormones involved in the regulation of reproductive functions. This mechanism of action makes Cetrorelix particularly effective in treating conditions related to the endocrine system and reproductive health.
The therapeutic areas of endocrinology and urogenital diseases encompass a wide range of conditions, and Cetrorelix's approval for these indications highlights its potential in addressing various medical needs. Endocrine system diseases can include disorders such as hormonal imbalances, while infertility in females refers to the inability to conceive or carry a pregnancy to term. Premature birth, on the other hand, refers to the delivery of a baby before the 37th week of pregnancy. Primary ovarian insufficiency is a condition characterized by the loss of ovarian function before the age of 40.
With its approval in multiple countries, including China, Cetrorelix has demonstrated its safety and efficacy in treating the indicated conditions. Its long history since the first approval in 1999 further establishes its track record in the pharmaceutical market. As a synthetic peptide drug targeting the GnRHR, Cetrorelix offers a promising option for patients suffering from endocrine system diseases, infertility, premature birth, and primary ovarian insufficiency.
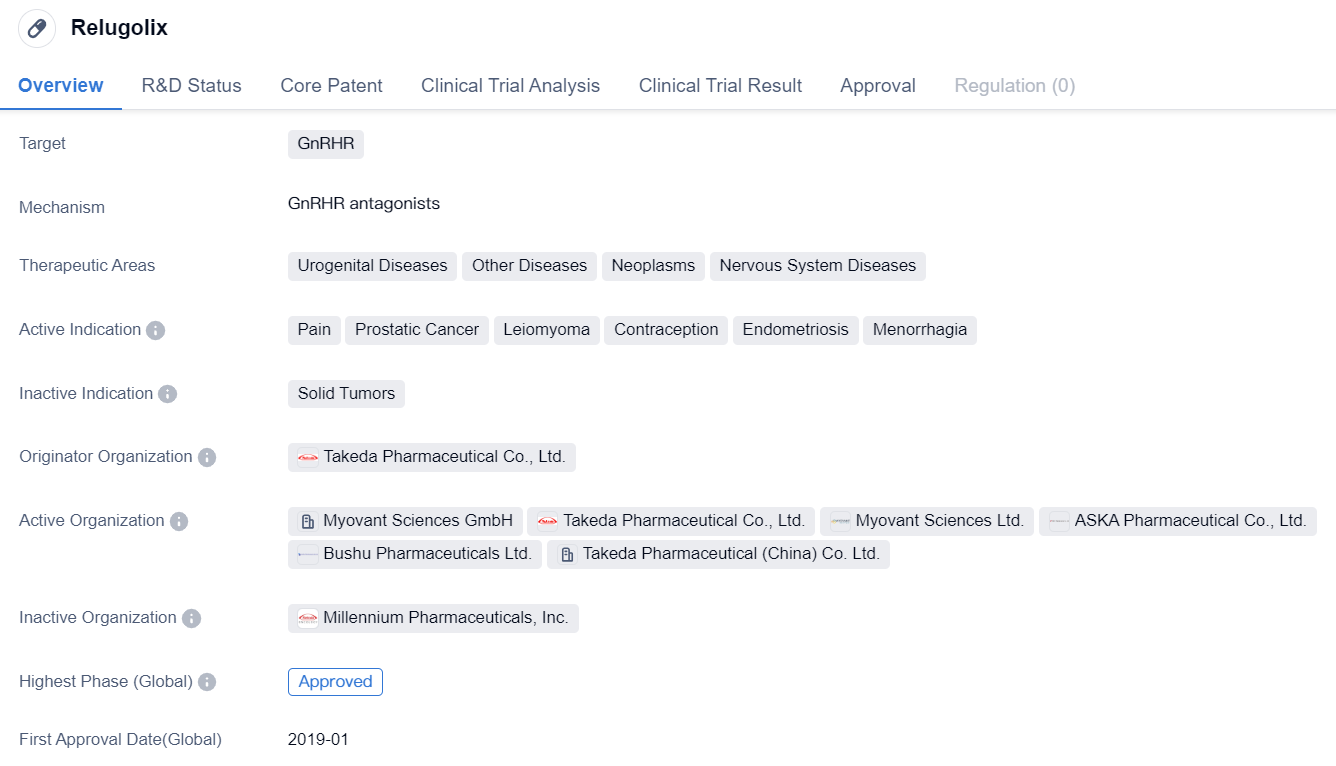
Relugolix
Relugolix is a small molecule drug that targets the GnRHR. It is primarily used in the treatment of various urogenital diseases, other diseases, neoplasms, and nervous system diseases. The drug has shown efficacy in managing pain, prostatic cancer, leiomyoma, contraception, endometriosis, and menorrhagia.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Relugolix was developed by Takeda Pharmaceutical Co., Ltd., a renowned pharmaceutical organization. It has successfully completed the highest phase of clinical trials globally and has received approval for commercial use. The drug was first approved in Japan in January 2019.
As a small molecule drug, Relugolix is designed to interact with the GnRHR, which plays a crucial role in regulating the release of reproductive hormones. By targeting this receptor, Relugolix can modulate hormone levels and provide therapeutic benefits in various disease conditions.
The therapeutic areas in which Relugolix is indicated suggest its potential in addressing a wide range of medical conditions. Urogenital diseases, such as prostatic cancer, leiomyoma (benign tumors of the uterus), and menorrhagia (excessive menstrual bleeding), can benefit from the drug's mechanism of action. Additionally, Relugolix shows promise in managing other diseases and neoplasms, indicating its potential application in diverse medical fields.
The drug's approval in Japan signifies its safety and efficacy profile, as assessed by regulatory authorities. This milestone highlights the successful completion of rigorous clinical trials and the drug's potential to address unmet medical needs in the indicated therapeutic areas.
While Relugolix has reached the highest phase of clinical trials globally, it is still in Phase 3 in China. This suggests that the drug is undergoing further evaluation and testing in the Chinese market, potentially paving the way for its future approval and commercialization in the country.
In summary, Relugolix is a small molecule drug developed by Takeda Pharmaceutical Co., Ltd. It targets the GnRHR and has shown efficacy in treating various urogenital diseases, other diseases, neoplasms, and nervous system diseases. The drug has been approved for commercial use in Japan and is currently in Phase 3 in China. Its approval and therapeutic indications highlight its potential to address unmet medical needs in multiple therapeutic areas.