ESSA Pharma starts Phase 2 trial of EPI-7386 with Enzalutamide for Metastatic Castration-Resistant Prostate Cancer patients
ESSA Pharma Inc., a company in the clinical phase of pharmaceutical operations with a focus on developing new treatments for prostate cancer, commenced the Phase 2 segment of its Phase 1/2 trial. The research is assessing its chief candidate, masofaniten (previously referred to as EPI-7386), a first-of-its-kind N-terminal domain androgen receptor inhibitor. It is tested in concert with Astellas and Pfizer's antiandrogen enzalutamide in individuals suffering from metastatic castration-resistant prostate cancer that have never been treated with second-generation antiandrogens.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Results from Phase 1 dose escalation in the first four patient cohorts of the ongoing Phase 1/2 investigation are set for a poster demonstration at the forthcoming European Society of Medical Oncology Congress, which runs from October 20-24, 2023, in Madrid, Spain.
David Parkinson, M.D., the President and CEO of ESSA, stated "The commencement of the randomised Phase 2 division of our research examining the collaboration of masofaniten and enzalutamide marks a crucial advancement for ESSA. We anticipate sharing revised results from the Phase 1 dose adjustment segment next month at ESMO 2023."
He added, "The observed favorable safety overview, thus far, with this Combo has guided the safety review board to consent us to proceed into the next comparative study portion using the dose regimen that was tried in the fourth cohort as the recommended Phase 2 dosage. We are eager to further clarify the potential of this Combo, towards enhancing the long-term clinical outcome for mCRPC patients. We intend to offer direction on when to publicly reveal initial data when Phase 2 has been in progress for several months."
Masofaniten is a pioneering, profoundly selective oral, small molecule drug that hampers the N-terminal domain ("NTD") of the androgen receptor ("AR"). At present, there is an ongoing open-label, randomised Phase 2 clinical investigation into the use of masofaniten in conjunction with enzalutamide for metastatic castration-resistant prostate cancer. Meanwhile, ESSA has also been carrying out a Phase 1 study using masofaniten as a single therapy for mCRPC patients whose tumors have been unresponsive to standard treatments.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
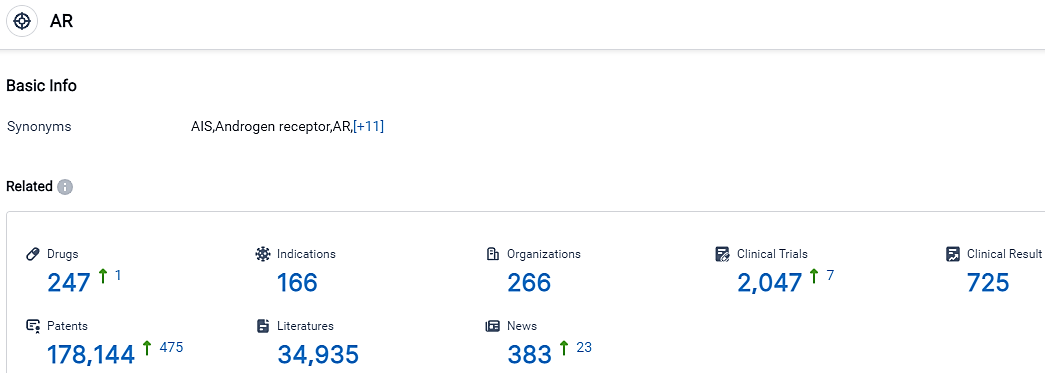
According to the data provided by the Synapse Database, As of September 20, 2023, there are 247 investigational drugs for the AR target, including 166 indications,266 R&D institutions involved, with related clinical trials reaching 2047,and as many as 178144 patents.
Masofaniten's unique mechanism of action disrupts the AR signaling pathway, the primary pathway that drives prostate cancer growth, by selectively binding to the NTD, a region of the AR that is not currently targeted by other therapies. The U.S. Food and Drug Administration has granted Fast Track designation to masofaniten for the treatment of adult male patients with mCRPC resistant to standard-of-care treatment. ESSA retains all rights to masofaniten worldwide.