Anaptys: Phase 2b Trial of BTLA Agonist ANB032 Fails in Atopic Dermatitis
AnaptysBio, Inc. (Nasdaq: ANAB), a biotechnology firm in the clinical development phase specializing in innovative immunology treatments, has announced that its experimental drug ANB032, which acts as a BTLA agonist, failed to achieve the primary and secondary objectives across all dose levels in the global ARISE-AD study involving 201 patients. This trial assessed ANB032 as a standalone treatment for moderate to severe atopic dermatitis (AD) or eczema. The drug was found to be well tolerated, with no safety concerns raised.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
"Although ANB032 has shown to be safe and well tolerated, we are disappointed by the efficacy outcomes in atopic dermatitis (AD) and will cease further investment in this asset. Going forward, we will concentrate our resources and capital on the remainder of our promising autoimmune portfolio," stated Daniel Faga, president and CEO of Anaptys. "PD-1 is a co-inhibitory receptor that is predominantly expressed on activated T cells. We are eager to provide updates on rosnilimab, a depleter and agonist aimed at PD-1+ T cells, with top-line Phase 2b data for rheumatoid arthritis anticipated in February 2025, and top-line Phase 2 data for ulcerative colitis expected in the first quarter of 2026, followed by Phase 1b data from two additional studies."
The ARISE-AD trial assessed the effectiveness, safety, tolerability, pharmacokinetics, and pharmacodynamics of ANB032 as a monotherapy in individuals with moderate to severe AD. A total of 201 patients with a mean baseline Eczema Area and Severity Index (EASI) score of 27.3 participated in the study conducted across the U.S., Canada, Europe, Australia, and New Zealand. Of these, 168 were biologics naïve and 33 had experience with biologics, defined as having received dupilumab or other IL-13 targeted therapies. Patients were randomized to receive either 100 mg of subcutaneous ANB032 every four weeks (Q4W), 400 mg every four weeks (Q4W), 400 mg every two weeks (Q2W), or a placebo for a duration of 12 weeks. The primary and secondary endpoints were evaluated at Week 14.
Regardless of their previous treatment history, ANB032 failed to meet the primary endpoint of achieving at least a 75% improvement from baseline in the EASI score (EASI-75) or any of the secondary endpoints at Week 14, including EASI-90, the mean change in baseline EASI, or a 4-point decrease in itch severity as evaluated by the peak Pruritus Numerical Rating Scale (PNRS) when compared to placebo.
The absolute response rates on significant endpoints for patients receiving ANB032 came close to meeting the minimum acceptable product profile with sustained off-drug responses; however, elevated placebo response rates that exceeded historical averages, particularly in the U.S., were noted.
ANB032 was well tolerated across all dosage levels, with no safety concerns identified. In alignment with previous investigations, the data indicated a positive safety and tolerability profile for ANB032; only one participant out of the three active dose groups experienced a serious adverse event (SAE) related to worsening AD, while two participants in the placebo group had SAEs. No correlation between dose and adverse events (AEs) was evident, and no safety signals were detected. The most frequently observed AEs (>5%) included nasopharyngitis, atopic dermatitis, and headache.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
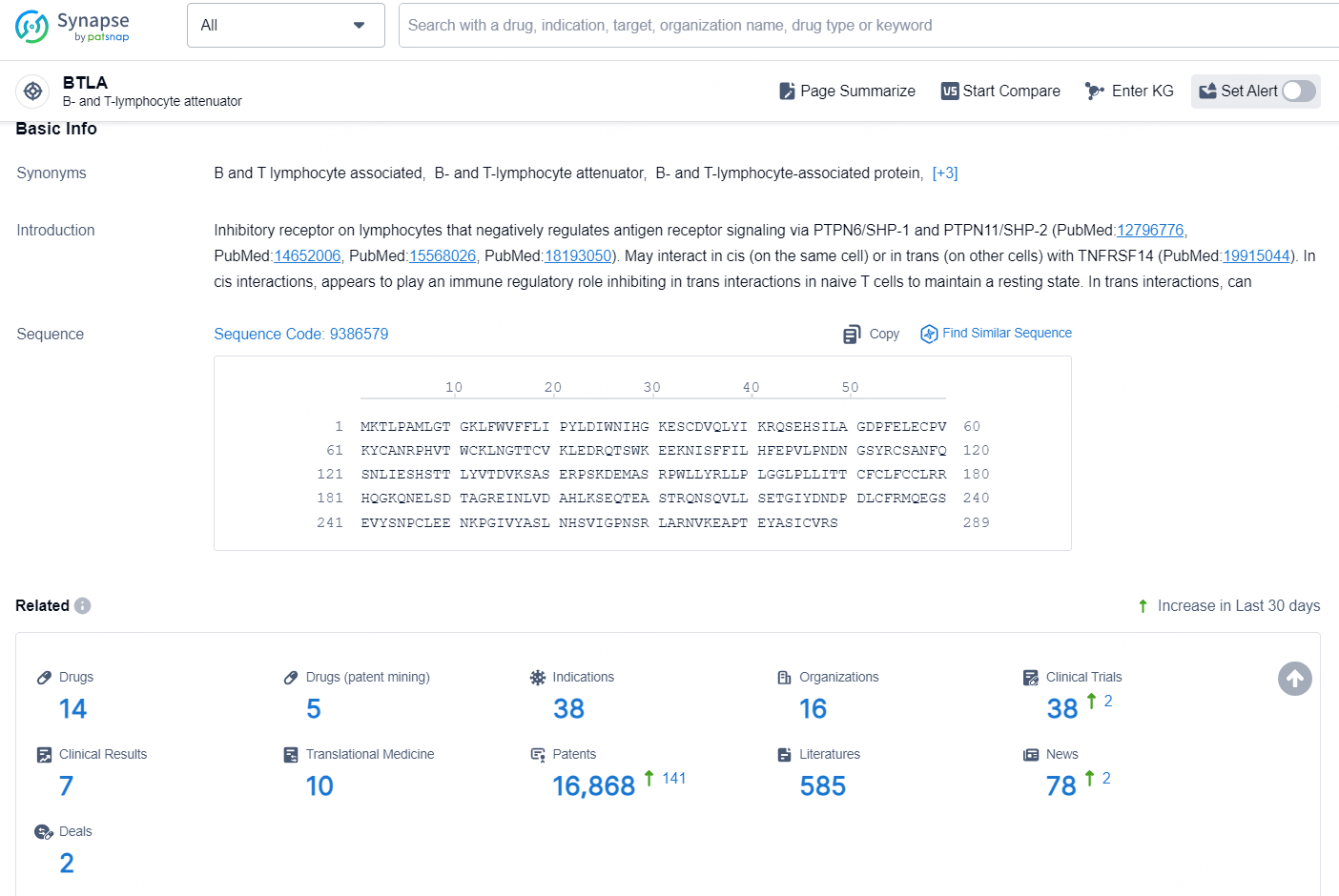
According to the data provided by the Synapse Database, As of December 18, 2024, there are 14 investigational drugs for the BTLA target, including 38 indications, 16 R&D institutions involved, with related clinical trials reaching 38, and as many as 16868 patents.
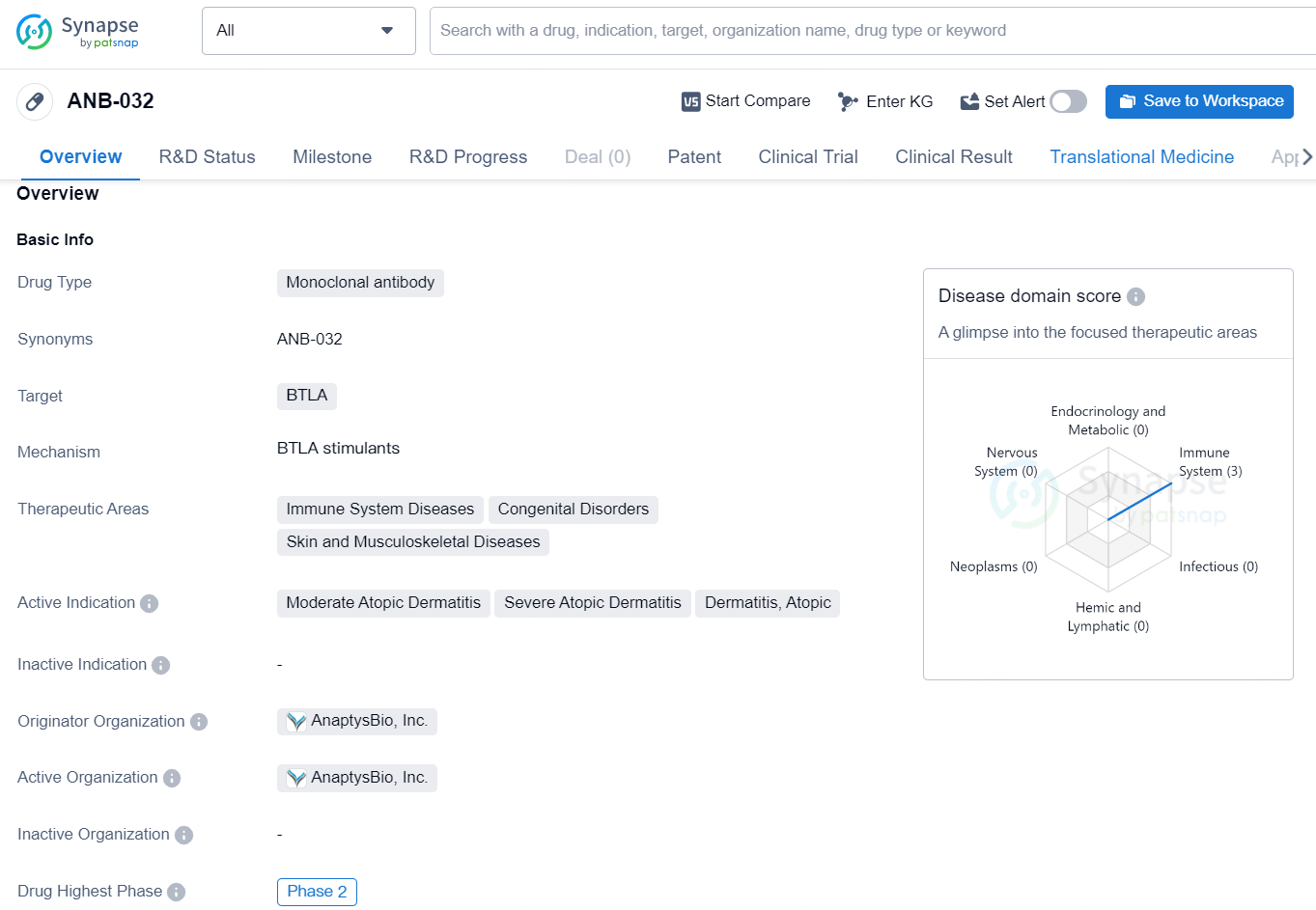
ANB-032 is a monoclonal antibody drug developed by AnaptysBio, Inc. The drug targets BTLA and is designed to treat a range of immune system diseases, congenital disorders, as well as skin and musculoskeletal diseases. The active indications for ANB-032 include moderate atopic dermatitis, severe atopic dermatitis, and dermatitis, atopic. Currently, ANB-032 is in the Phase 2 of clinical trials, which is the highest phase it has reached globally.