Leriglitazone Meets Key Goals in NEXUS Trial for Cerebral ALD in Children
Minoryx Therapeutics, a biotechnology firm in the late stages of development specializing in treatments for rare central nervous system (CNS) conditions, along with Neuraxpharm Group (Neuraxpharm), a prominent European specialty pharmaceutical company dedicated to addressing CNS disorders, has announced the completion of the NEXUS trial, achieving its primary objective. Both organizations plan to submit a request for European Marketing Authorization for leriglitazone aimed at pediatric and adult patients with cerebral Adrenoleukodystrophy (cALD) by the middle of 2025.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The NEXUS trial was a significant, 96-week, open-label research study aimed at assessing the efficacy and safety of leriglitazone administered orally once daily in pediatric patients diagnosed with cALD. The primary outcome measure focused on the percentage of participants who exhibited clinical and radiological disease stabilization by week 96 or during a visit before undergoing hematopoietic stem cell transplant (HSCT). The analysable cohort consisted of 20 patients who received treatment for at least 24 weeks. Throughout the treatment period, all 20 patients remained clinically stable, with 7 patients (35%) fulfilling the criteria for disease stabilization, which is markedly higher than the 10% incidence of self-arrest that would be predicted based on natural history data (p<0.05).
Leriglitazone was well tolerated among all pediatric participants, with no serious adverse events attributed to the treatment and no discontinuations due to treatment-related side effects.
The comprehensive findings from the NEXUS trial are scheduled to be shared at upcoming neurology conferences.
“Cerebral ALD presents a serious challenge for affected boys and their families. Treatment options are scarce, and there is an ongoing search among healthcare providers and families for improved therapies to manage cALD," stated Patricia Musolino, Global Principal Investigator of the NEXUS study. “The findings from NEXUS confirm that leriglitazone meets a critical need for non-invasive therapies that can be initiated as soon as brain lesions are detected, potentially halting or slowing lesion progression.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
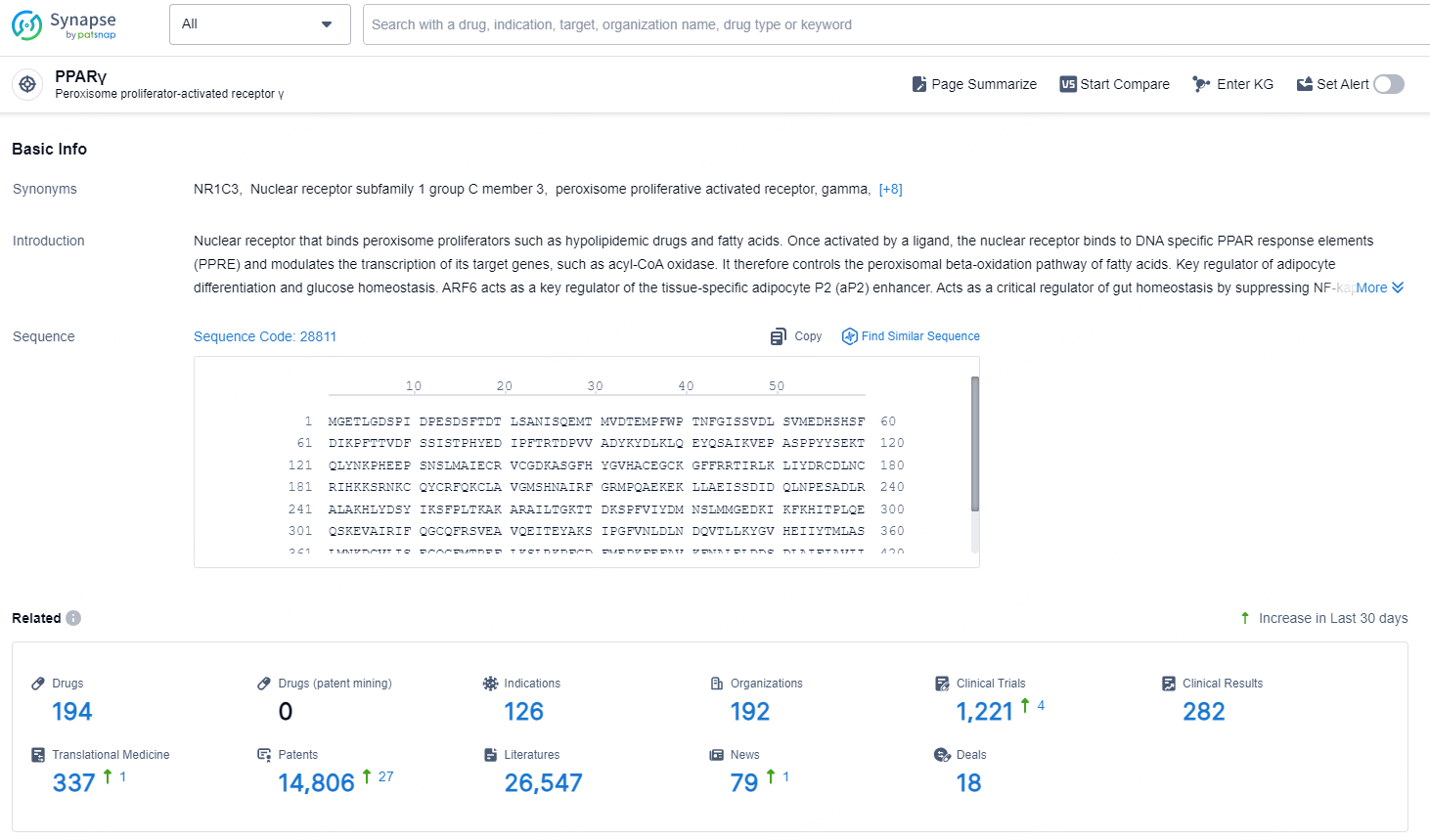
According to the data provided by the Synapse Chemical, As of December 17, 2024, there are 194 investigational drugs for the PPARγ target, including 126 indications, 192 R&D institutions involved, with related clinical trials reaching1221, and as many as 14806 patents.
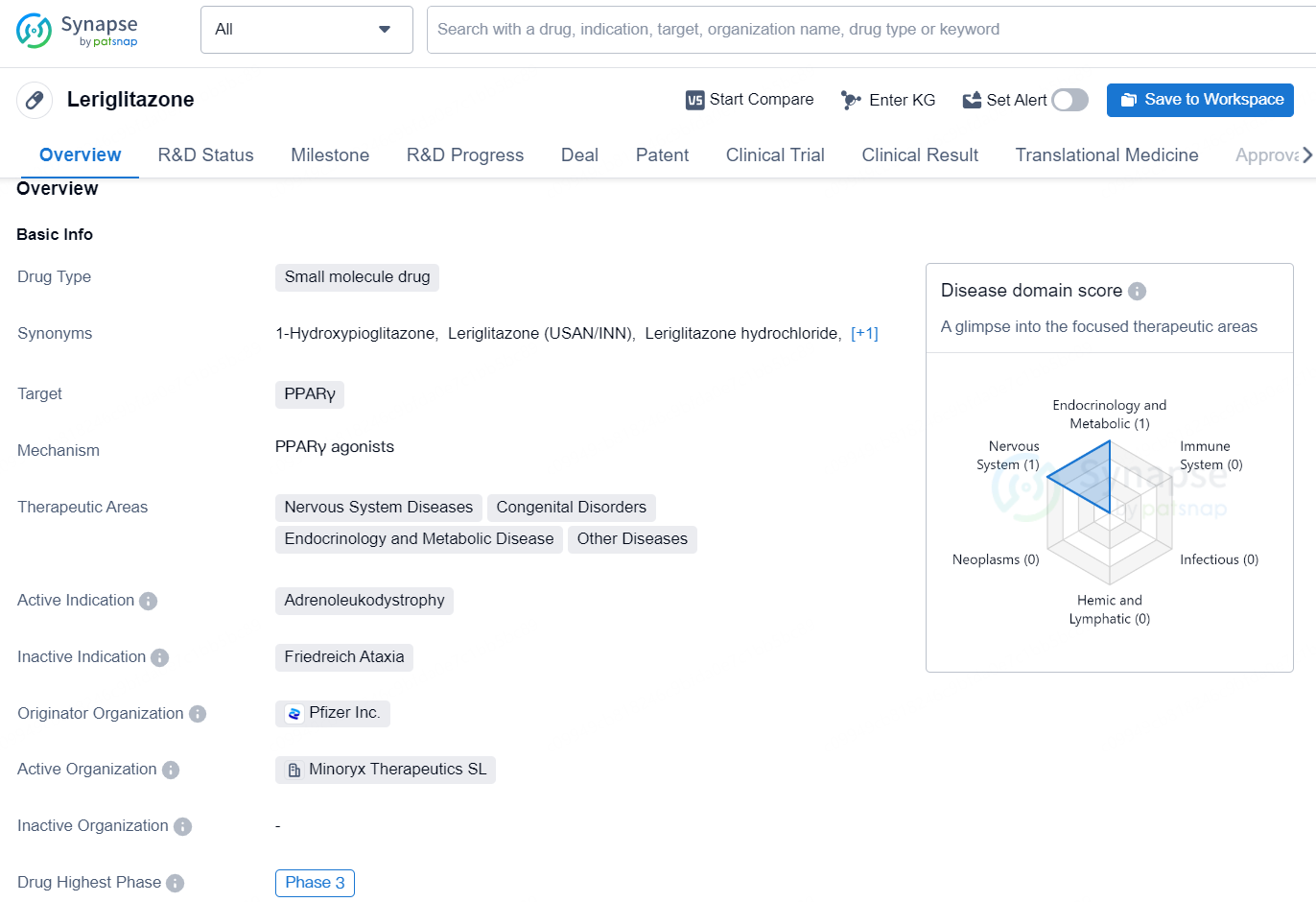
Leriglitazone is a small molecule drug that targets PPARγ and is being developed for the treatment of various therapeutic areas including Nervous System Diseases, Congenital Disorders, Endocrinology and Metabolic Disease, and Other Diseases. Its active indication is Adrenoleukodystrophy. The drug is being developed by Pfizer Inc. and has reached the highest phase of development, which is Phase 3, on a global scale.