Zealand Pharma Begins Phase 2b ZUPREME-1 Trial for Petrelintide in Overweight/Obesity
Zealand Pharma A/S (Nasdaq: ZEAL), a biopharmaceutical firm dedicated to innovative peptide-based therapies, has announced the enrollment of the first participant in ZUPREME-1. This global Phase 2b study focuses on individuals with obesity or those who are overweight and suffering from weight-related co-morbidities. The trial evaluates the effects of petrelintide, a long-acting amylin analog administered subcutaneously once a week, compared to a placebo, particularly in terms of body weight outcomes, safety, and tolerability.
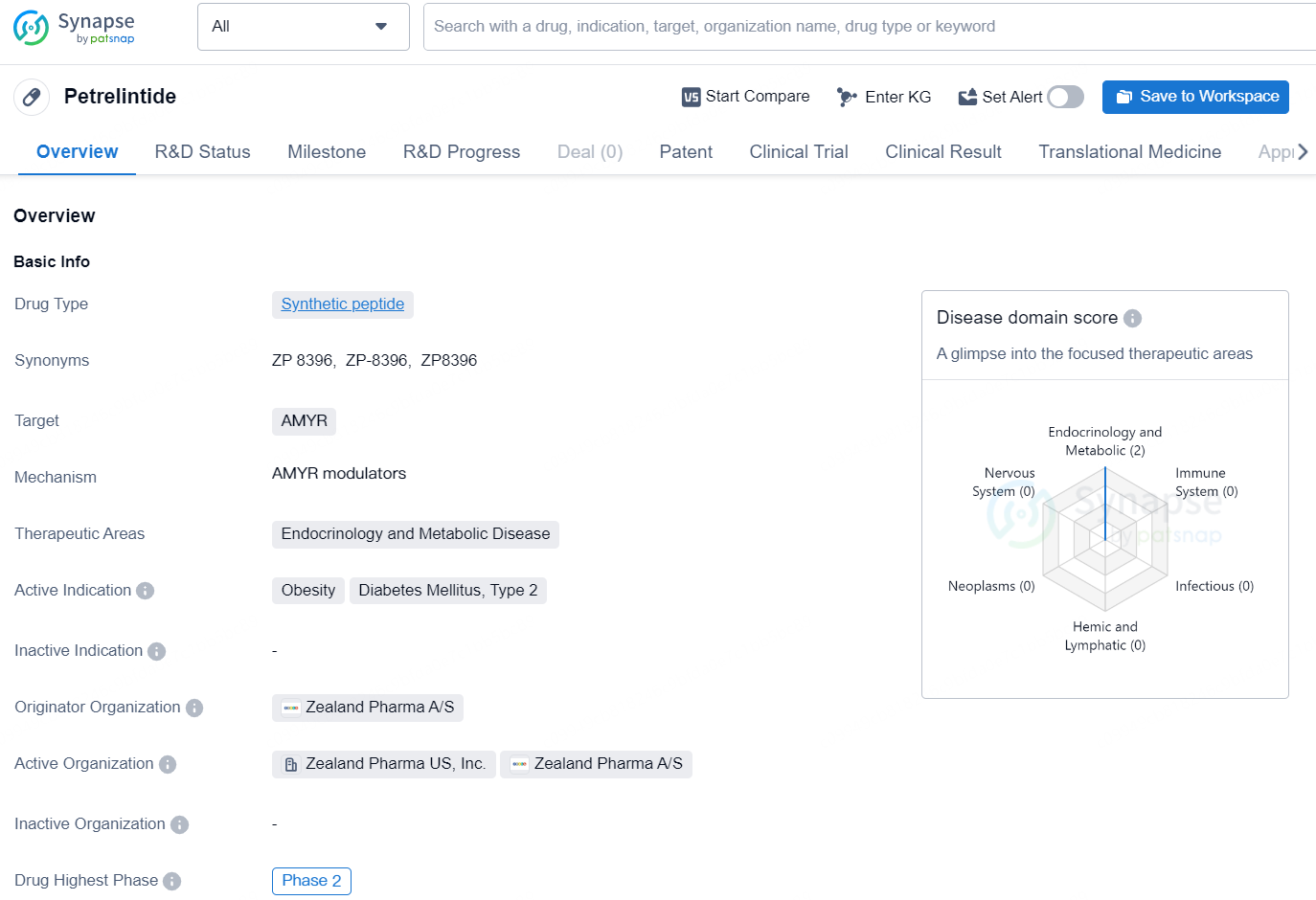
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"The launch of the extensive Phase 2b study of petrelintide in individuals who are overweight or obese signifies a key achievement for Zealand. Supported by robust and persuasive clinical evidence gathered so far, we are optimistic about petrelintide's potential to become a leading option among incretin-based treatments and a future mainstay in weight management,” stated David Kendall, MD, Chief Medical Officer at Zealand Pharma. “We are broadening the development strategy for petrelintide and anticipate starting the Phase 2b trial in people with overweight or obesity accompanied by type 2 diabetes in the first half of 2025."
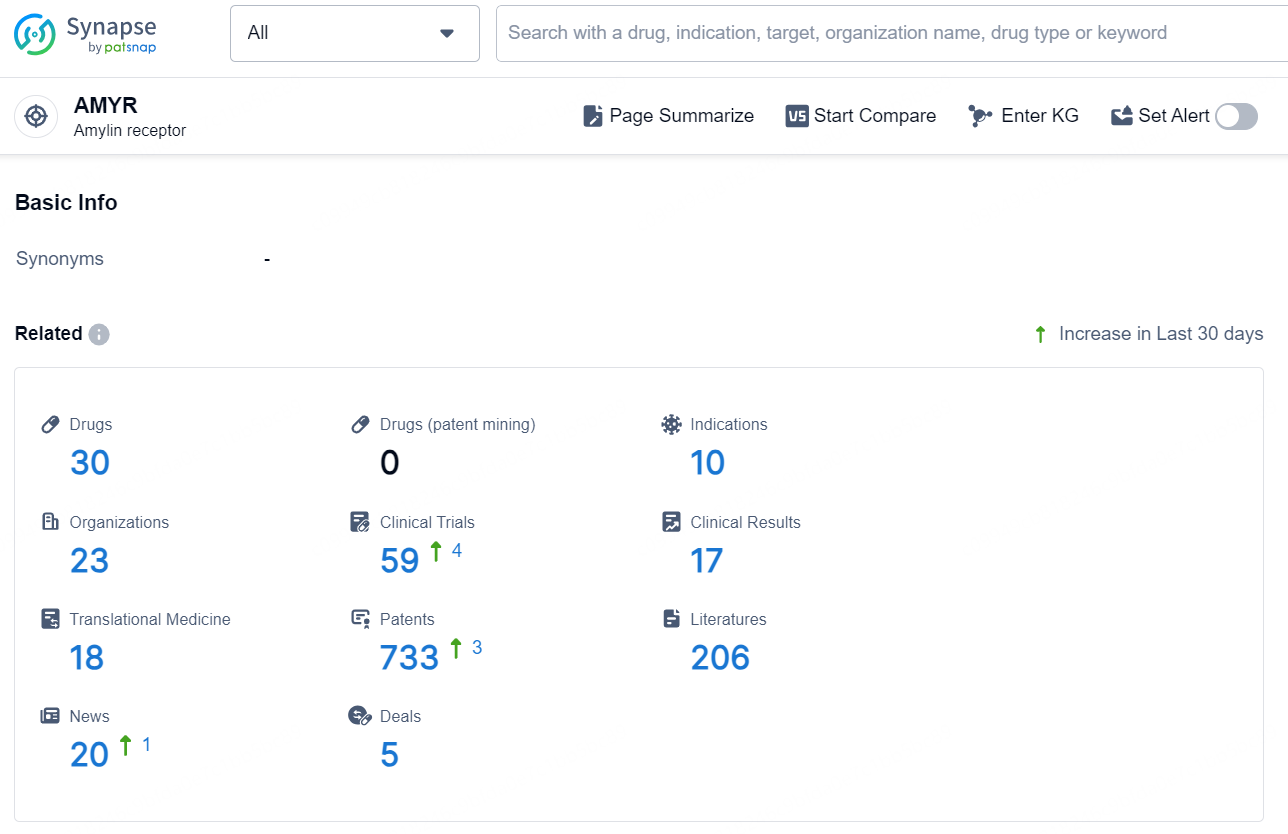
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of December 17, 2024, there are 30 investigational drugs for the AMYR target, including 10 indications, 23 R&D institutions involved, with related clinical trials reaching 59, and as many as 733 patents.
Petrelintide is a synthetic peptide drug developed by Zealand Pharma A/S, targeting the AMYR receptor. The drug falls under the therapeutic areas of endocrinology and metabolic disease, specifically aimed at treating obesity and diabetes mellitus type 2. As of the latest available information, Petrelintide has reached the highest global phase of development at Phase 2.