AnaptysBio Announces Positive Early Results from Phase 3 GEMINI-2 Study of Imsidolimab for Generalized Pustular Psoriasis
AnaptysBio, Inc., a biotech firm in the clinical phase that specializes in developing novel treatments for immunological conditions, has revealed encouraging preliminary outcomes from the international Phase 3 studies, GEMINI-1 and GEMINI-2. These studies were conducted to assess the effectiveness and safety of the investigational drug imsidolimab (IL-36R mAb) in individuals suffering from generalized pustular psoriasis. This condition, an aggressive and rare orphan disease, can become critical if not properly managed.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
In the GEMINI-1 Phase 3 study involving 45 patients, the participants were divided into three groups with a 1:1:1 ratio: one group received a solitary 750mg dose of intravenous imsidolimab, the second received 300mg of the same drug intravenously, and the third group was given a placebo on Day 0. Results showed that 53% of the individuals administered with a single 750mg dose of IV imsidolimab reached a GPP Physician Global Assessment score of 0/1 by the fourth week, whereas only 13% of those on placebo achieved the same score. Similarly, 53% of those receiving a single 300mg IV dose of imsidolimab also reached a score of 0/1 at Week 4.
Subsequently, in the GEMINI-2 Phase 3 trial, 16 patients who responded with a GPPPGA score of 0/1 in the GEMINI-1 trial were reassigned either to a monthly regimen of 200mg subcutaneous imsidolimab or a placebo. These patients were monitored for a minimum of 24 weeks and up to a maximum of 92 weeks. Among the eight individuals reallocated to the monthly 200mg SC imsidolimab, every single one sustained the GPPPGA score of 0/1 without flare-ups. Conversely, of those shifted to placebo, only 25% maintained the GPPPGA score of 0/1, and 63% suffered from flare-ups.
Daniel Faga, the president and CEO of Anaptys, commented, “The GEMINI-1 and GEMINI-2 Phase 3 trials exemplify Anaptys's expertise in in-house discovery and development of unique antibodies that significantly improve patient outcomes.” He further noted, “These moderately scaled studies established that a single IV dose of imsidolimab suffices to substantially clear GPP within four weeks with maintenance achieved through monthly SC doses. Our focus continues to be on licensing imsidolimab to make this treatment accessible to those afflicted by this severe disease.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
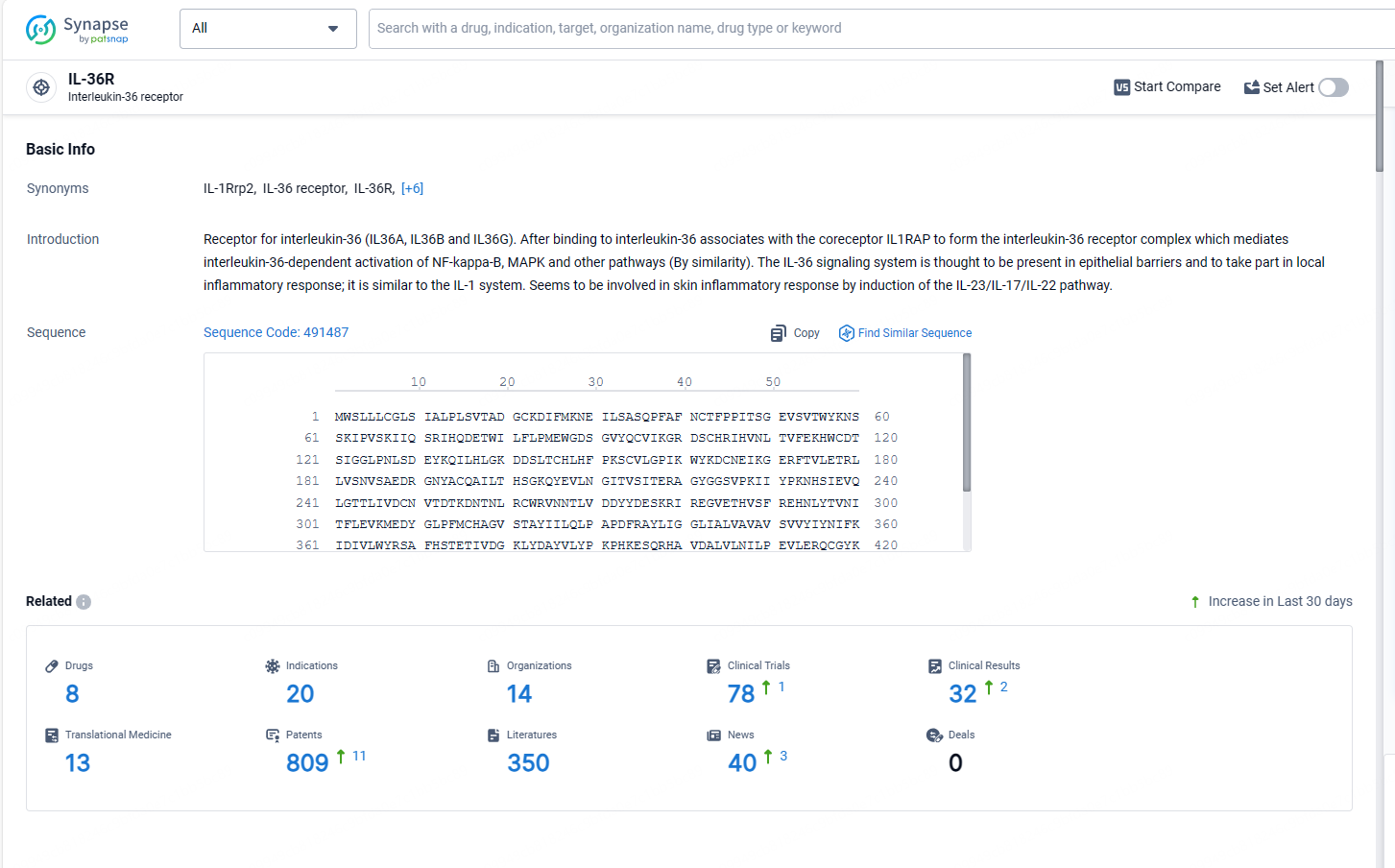
According to the data provided by the Synapse Database, As of May 13, 2024, there are 8 investigational drugs for the IL-36R target, including 20 indications, 14 R&D institutions involved, with related clinical trials reaching 78, and as many as 809 patents.
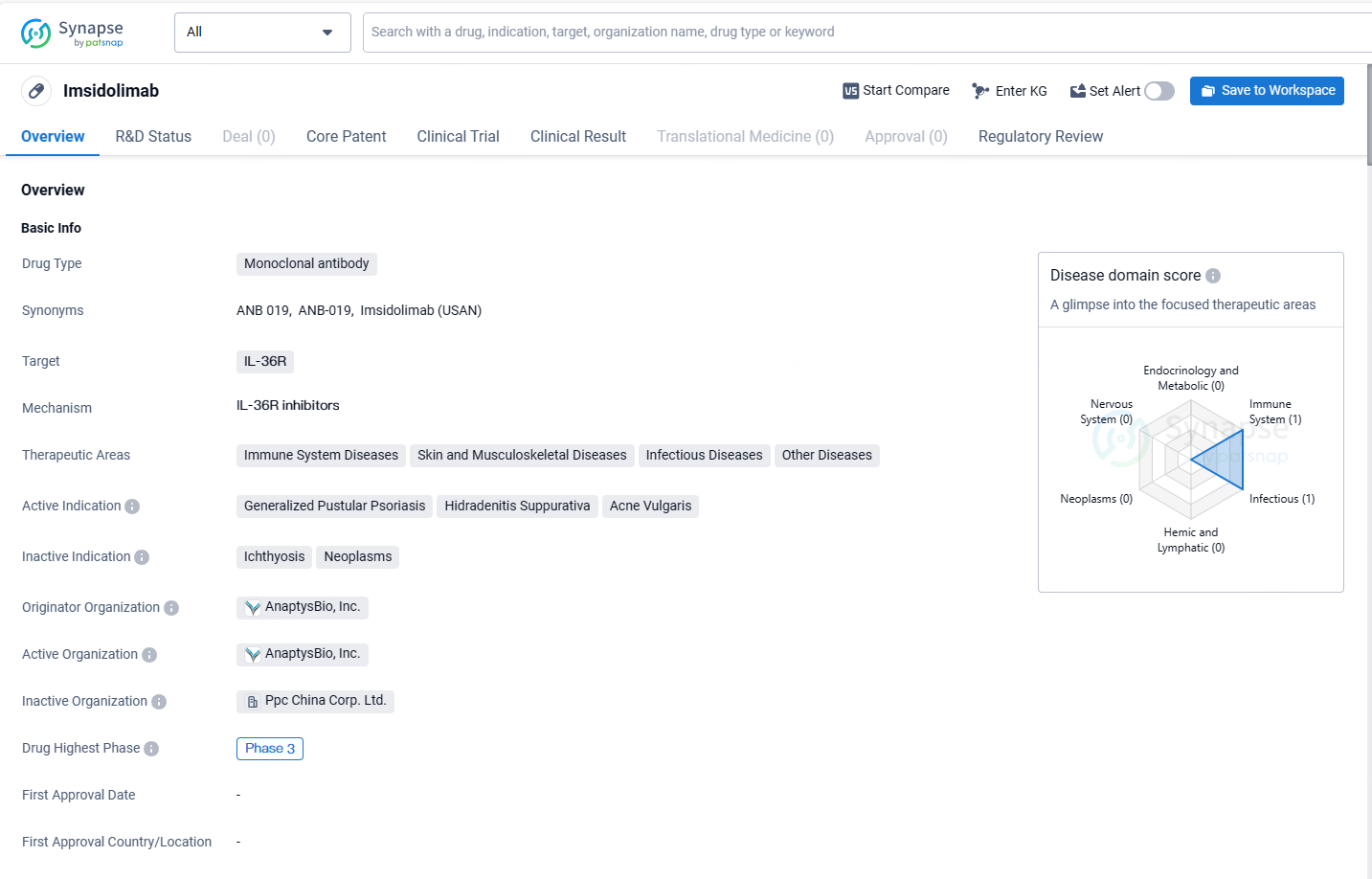
imsidolimab targets IL-36R and is being developed for the treatment of immune system diseases, skin and musculoskeletal diseases, infectious diseases, and other diseases. The active indications for imsidolimab include generalized pustular psoriasis, hidradenitis suppurativa, and acne vulgaris. It is currently in Phase 3 of clinical development globally, but its development in China has been discontinued at the highest phase. Imsidolimab has been designated as an orphan drug, providing certain benefits and incentives to the drug developer.