Antitumor Target: CCR8 Research Status
In January, Domain Therapeutics, a biopharmaceutical company focused on the development of innovative drugs targeting GPCRs, announced that it had successfully secured funding from the "Hospital-University Health Research SPRINT Alliance" project. This project will receive almost 10 million euros in financial support from the French National Research Agency for the advancement of precision medicine, including the progression of Domain's lead candidate drug DT-7012 to the clinical trial stage.
The SPRINT project aims to completely revolutionize the treatment paradigm for patients with Cutaneous T-Cell Lymphoma (CTCL), striving to provide a new curative paradigm as a standard treatment method. CTCL, a rare disease, affects about one in every 100,000 adults globally each year, with the incidence rate nearly tripling in the past 30 years. The impact on the quality of life for patients is profound, and the median overall survival for patients in the late stages is less than five years. Currently, the mechanisms of disease progression are not well understood.
It is reported that Domain had previously nominated a new drug candidate, DT-7012, a monoclonal antibody against the CCR8 receptor, which can effectively deplete tumor-infiltrating regulatory T cells (Tregs) and possesses great potential to become the best-in-class therapy in CTCL and solid tumors. The company targets highly strategic goals to develop novel immunotherapies that can improve therapeutic success rates in non-responsive cancer patients. In addition, the company has multiple therapeutic drugs targeting GPCRs in its development pipeline.
Domain Therapeutics and the SPRINT Alliance will undertake a trinitarian research strategy based on previous in-depth explorations into the CTCL tumor microenvironment. This includes:
1. Implementing a novel strategy for improved early non-biased predictions through the synergistic action of domain-driven and data-driven artificial intelligence
2. Validating molecular biomarkers for monoclonal antibody (mAb) responses
3. Innovating in drug development, targeting tumor cells and Tumor Microenvironment (TME) mechanisms resistant to mAbs, to provide long-term efficacy in mAb therapies.
Current Status of Targeted CCR8 Antibody Development
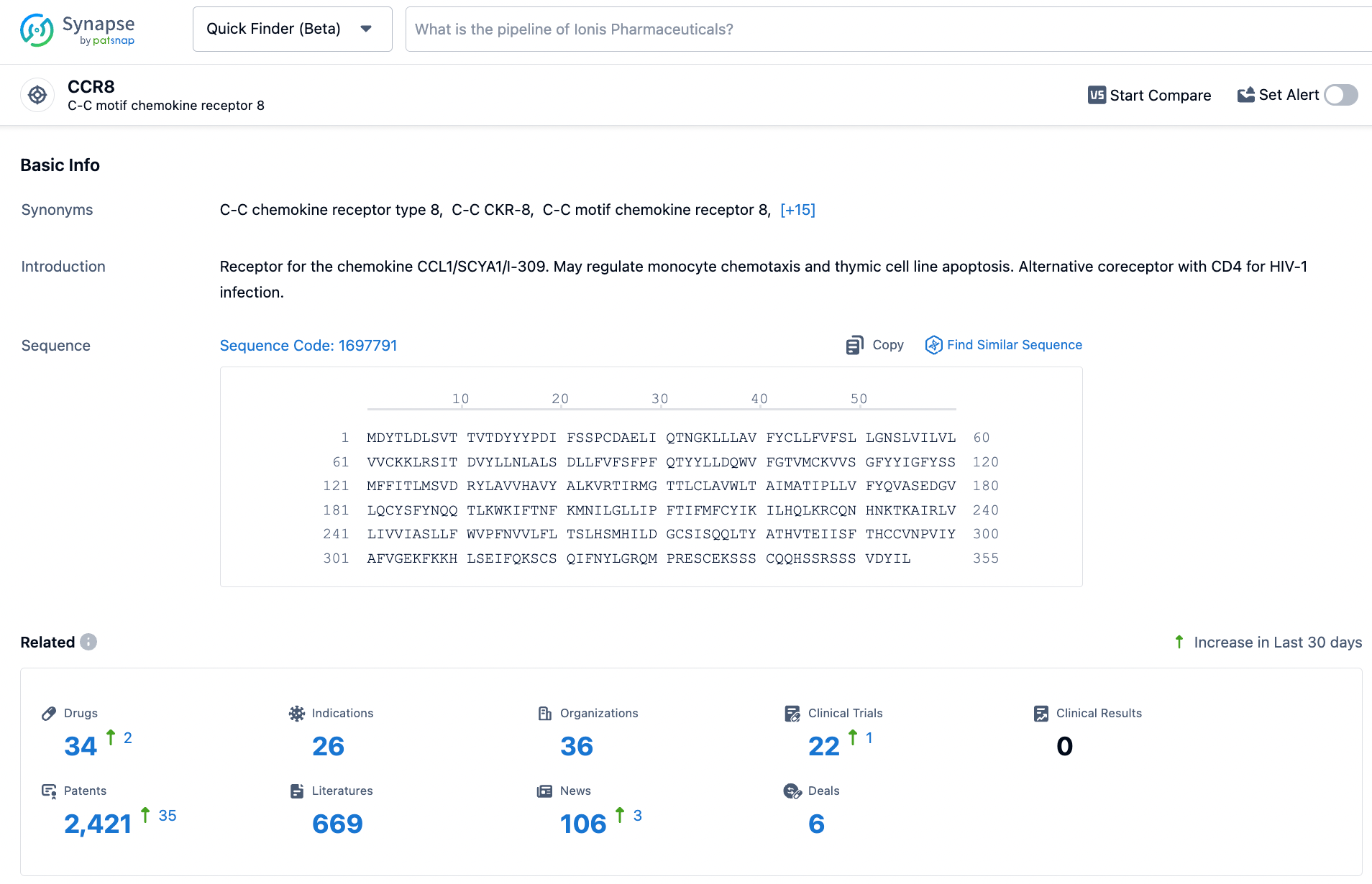
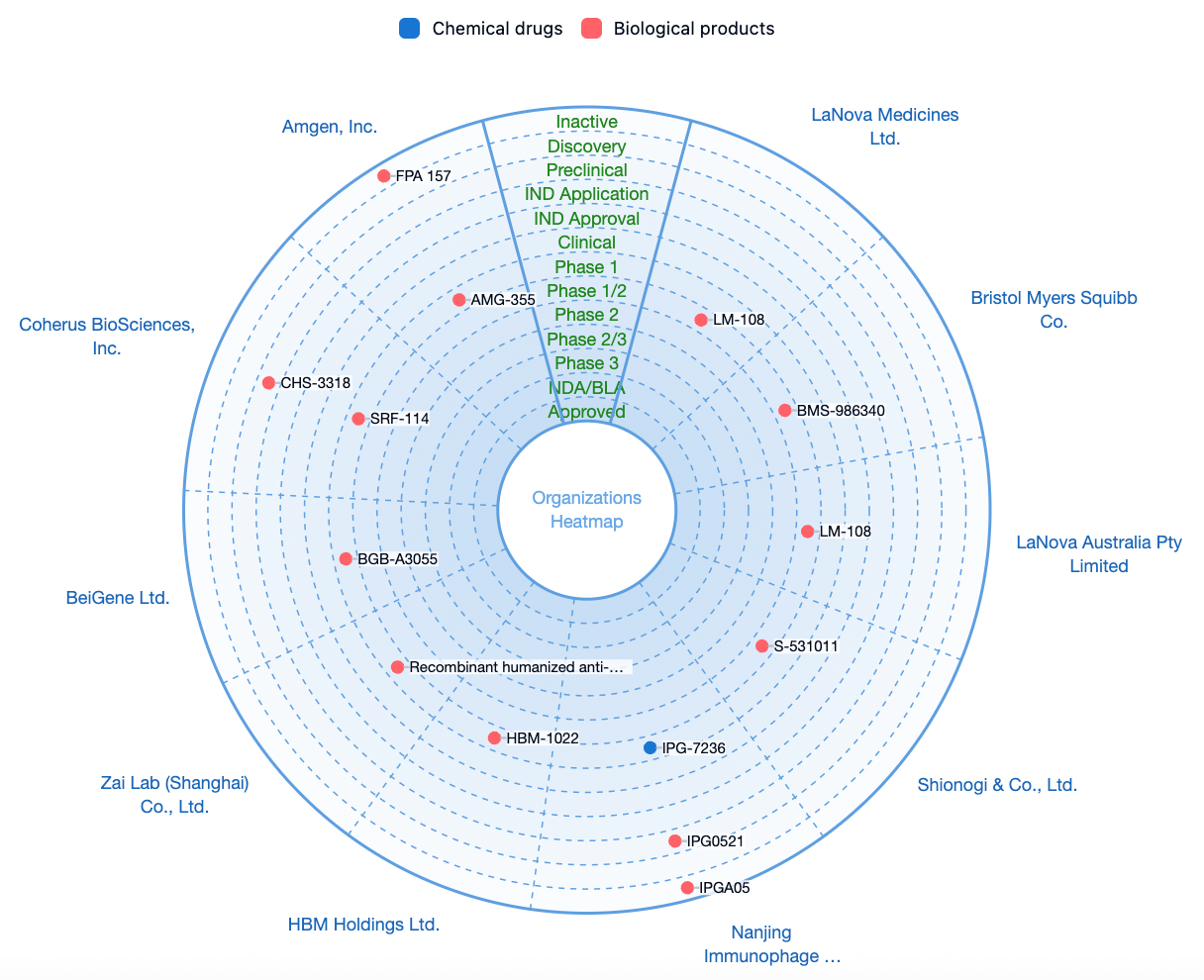
According to statistics from the Synapse database, there are currently approximately 34 disclosed research and development pipelines for CCR8 monoclonal antibodies, bispecific antibodies, and ADC (antibody-drug conjugates) worldwide. Among these, there are 3 in Phase 1/2 clinical trials, 11 in Phase 1 clinical trials, and 18 at the preclinical and drug discovery stages.
In the J.P. Morgan 2024 conference, the clinical pipelines related to CCR8 were mentioned by several companies, including BeiGene, Gilead, BMS (Bristol Myers Squibb), and Bayer, among others.
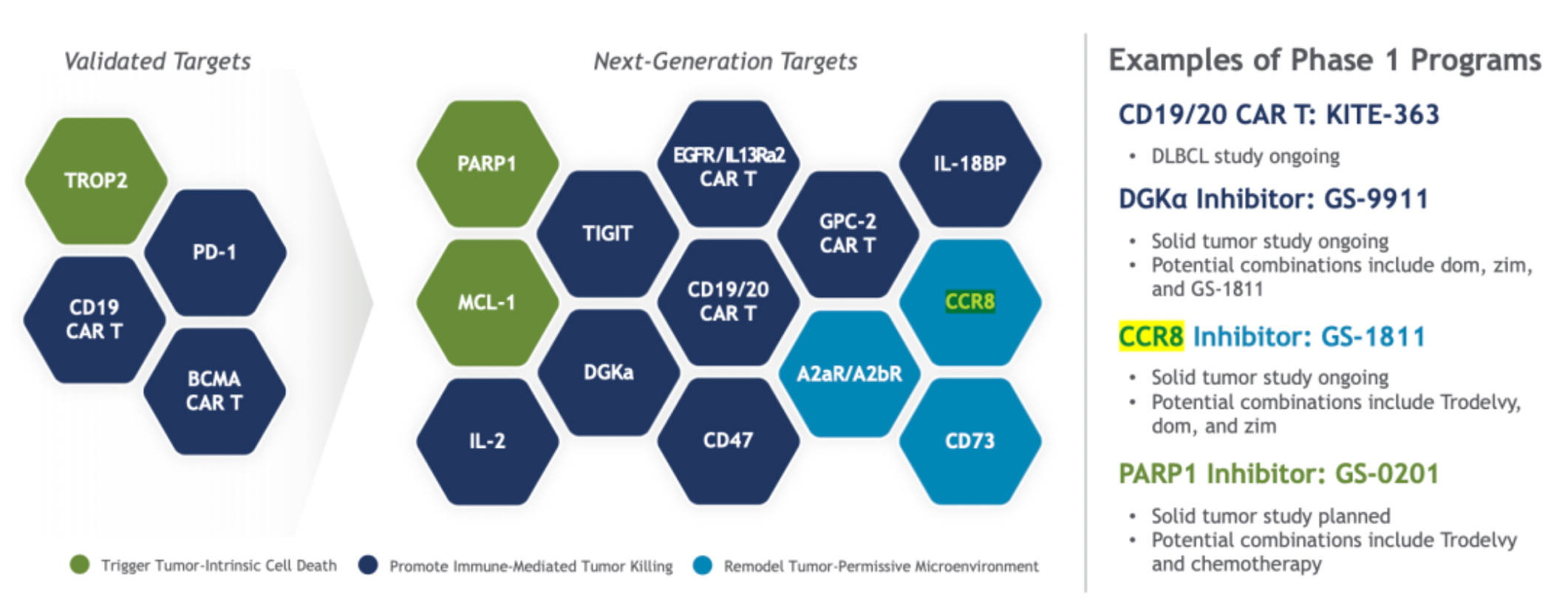
As part of Gilead's future plans, CCR8 is one of the next-generation targets of cancer research, which, alongside validated targets like PD-1, TROP2, BCMA, will form a comprehensive product line with combination potential for Gilead.
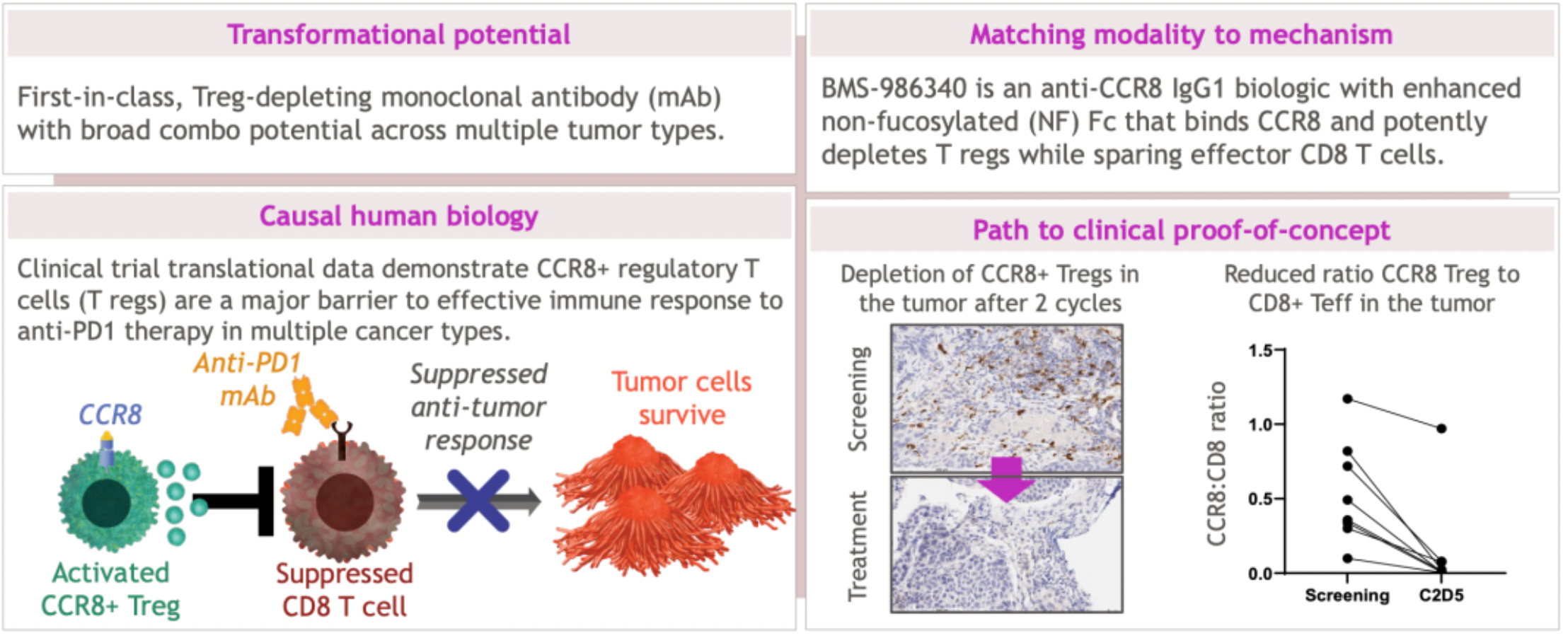
In the pipeline update previously provided by BMS, clinical trial data have indicated that CCR8+ regulatory T cells (Tregs) are major obstacles to effective immune responses to PD-1 inhibitor therapies across various cancer types. BMS-986340, an anti-CCR8 IgG1 biologic, features an enhanced afucosylated Fc region, which can specifically bind to CCR8 and efficiently deplete Tregs while sparing effector CD8+ T cells.