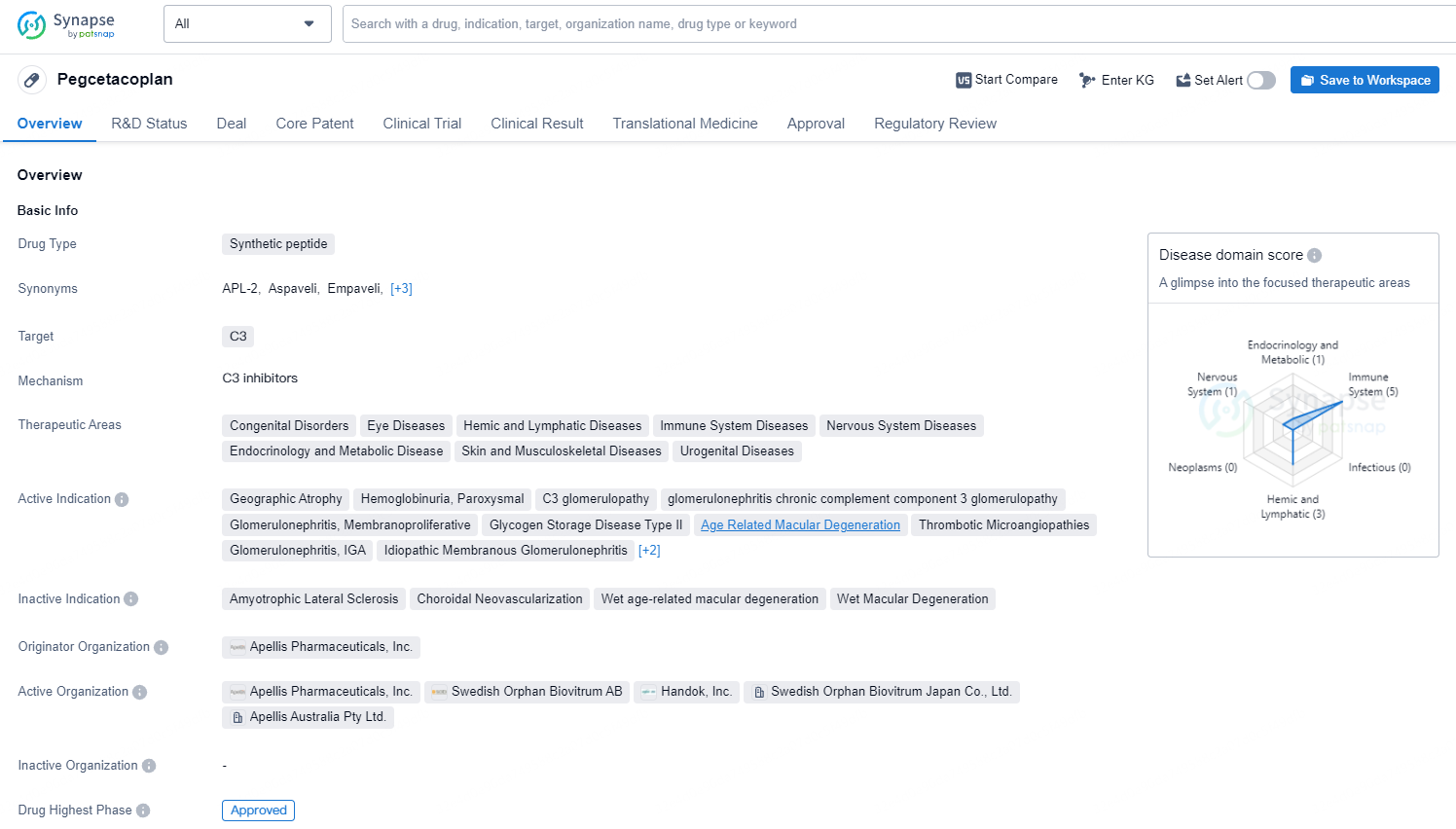
Aspaveli® (pegcetacoplan) received approval in Europe for new adult PNH patients
Sobi has revealed that the European Commission has granted approval for a new indication of Aspaveli® (pegcetacoplan) to treat adult individuals experiencing paroxysmal nocturnal haemoglobinuria with accompanying haemolytic anaemia.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Aspaveli has been sanctioned in Europe for the treatment of adult PNH patients who remain anaemic despite being on a C5 inhibitor for a minimum of three months. It has now become the first C3 inhibitor to be authorized as a primary treatment for PNH in Europe, significantly enhancing haemoglobin levels and other vital clinical indicators through its distinctive mechanism of action.
“The sanction of Aspaveli is anchored in compelling clinical evidence of its effectiveness and safety, providing medical practitioners and patients with more alternatives to manage PNH effectively,” commented Lydia Abad-Franch MD, who serves as the Head of R&D and Medical Affairs, and as Chief Medical Officer at Sobi.
Lydia Abad further stated, “With this approval, patients in Europe can commence Aspaveli treatment upon diagnosis or opt to switch from existing C5 inhibitor therapies should they exhibit signs of haemolytic anaemia. This development is significant in our commitment to enhance the therapeutic landscape for individuals grappling with this severe and intricate disorder.”
PNH is an uncommon, enduring, and life-endangering hematologic condition marked by unregulated complement activation that results in the breakdown of red blood cells that carry oxygen. Defined by continuously reduced haemoglobin levels, PNH often necessitates regular transfusions and can lead to severe exhaustion due to anaemia. Despite advancements with C5 inhibitor therapies, cross-sectional studies in the US and EU indicate that as many as 86% of individuals with PNH receiving C5 inhibitors continue to suffer from anaemia.
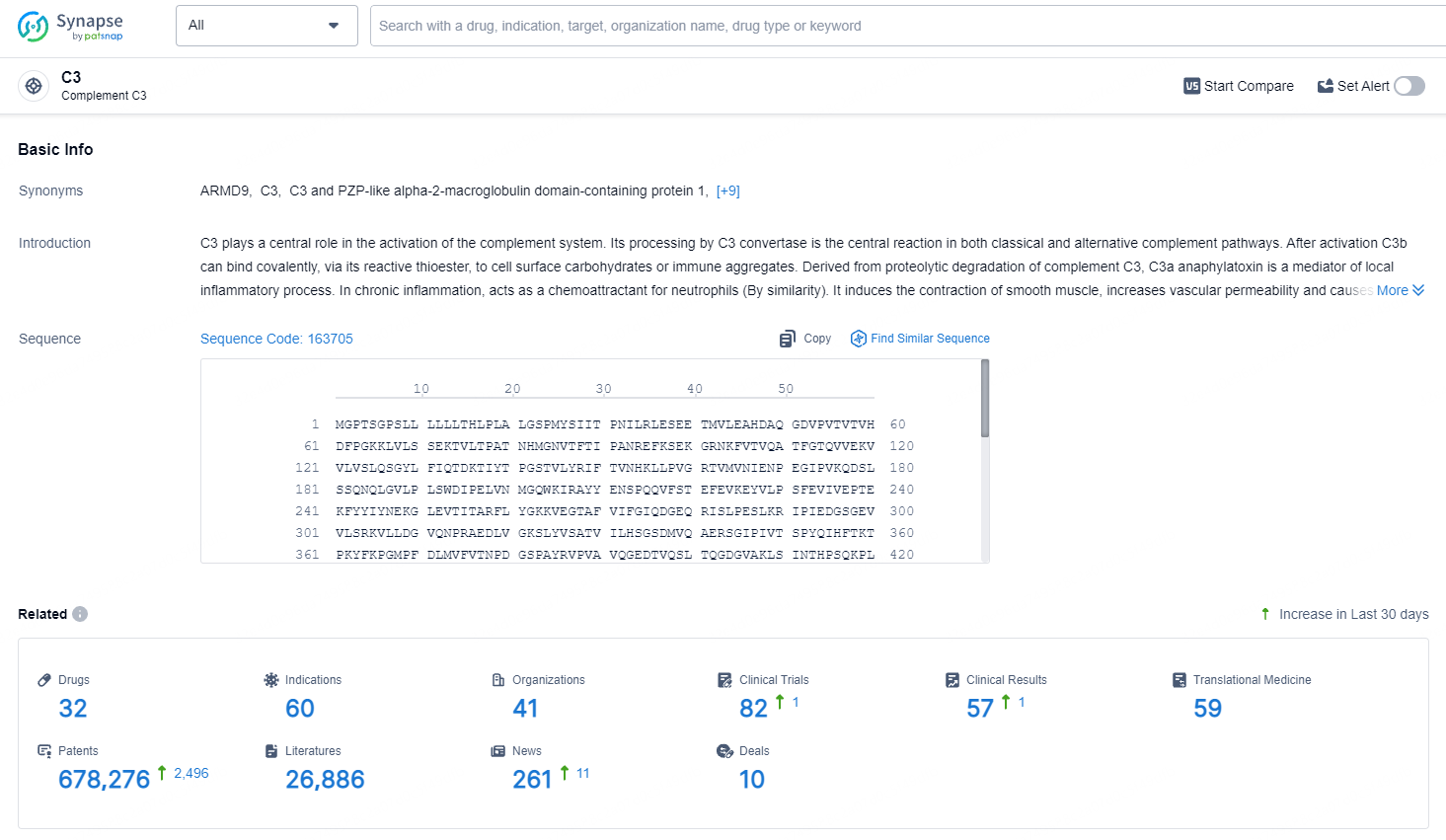
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of May 11, 2024, there are 32 investigational drugs for the C3 target, including 60 indications, 41 R&D institutions involved, with related clinical trials reaching 82, and as many as 678276 patents.
pegcetacoplan targets the C3 protein and has a broad range of therapeutic areas. The drug has received approval in the United States and is currently pending approval in China. Its first approval date was in May 2021, and it has been designated as a priority review, fast track, and orphan drug. Pegcetacoplan shows promise in addressing various diseases and has the potential to provide significant benefits to patients in need.