Neurocrine Biosciences Launches Phase 1 Trial to Assess NBI-1076986 in Healthy Participants
Neurocrine Biosciences, Inc. has launched its Phase 1 clinical trial, marking the first time humans are being evaluated. This study is aimed at assessing the safety, tolerability, pharmacokinetics, and pharmacodynamics of the experimental drug NBI-1076986 in healthy adult subjects.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
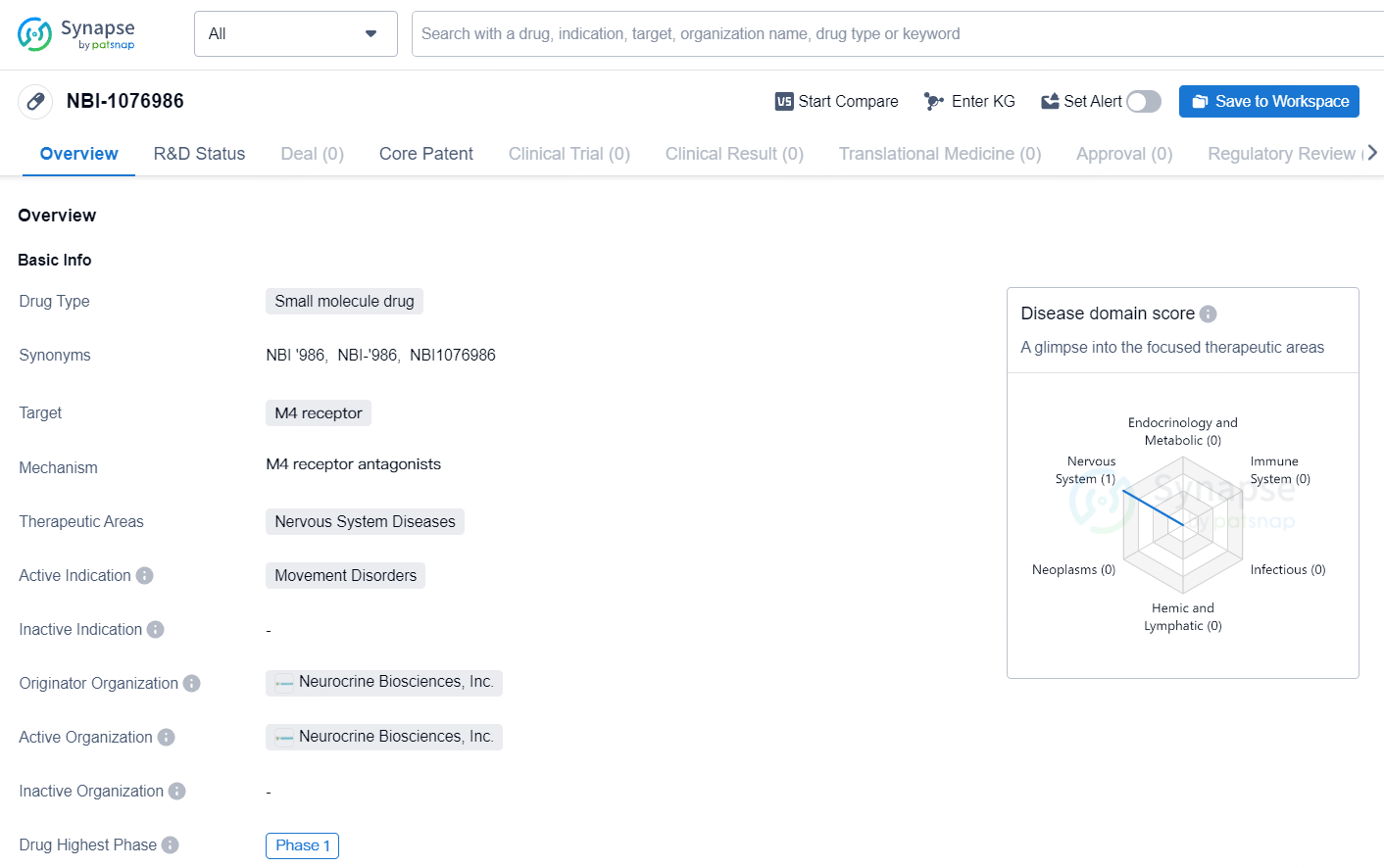
NBI-1076986 is a developmental, orally administered, M4 subtype-specific muscarinic acetylcholine receptor antagonist aimed primarily at treating various movement disorders, a formulation brought forth by Neurocrine Biosciences.
"Eiry W. Roberts, M.D., Chief Medical Officer at Neurocrine Biosciences, mentioned, “This compound is a crucial component of our targeted muscarinic portfolio, advancing into clinical trials as a promising therapy for movement disorders.” He further added, “Focusing specifically on M4 receptors might provide therapeutic gains for a wide array of motor coordination impairments, such as tremors in Parkinson’s disease and dystonia.”
Muscarinic receptors are integral for initiating signaling pathways in the brain. Among the five types involved in neurotransmission, M4 receptors are linked to motor coordination and some central nervous system disorders. Conversely, M1 receptors are involved in complex cognitive functions like learning and memory, and they are allegedly linked to the memory/cognitive complications seen with non-selective antimuscarinic drugs.
Introduced and perfected by Neurocrine Biosciences, NBI-1076986 is a novel, oral, M4 subtype-specific muscarinic acetylcholine receptor antagonist being evaluated for its efficacy in managing certain movement disorders such as Parkinson’s disease tremor and dystonia. The premise is that an M4-specific antagonist could better manage motor symptoms and movements, potentially reducing unintended effects.
The array of initiatives at Neurocrine Biosciences includes a diverse array of muscarinic receptor-targeting programs. Beyond NBI-1076986, the company's collection comprises several small molecule M1, M1/M4, and M4 agonists following an agreement to develop and market these from Nxera Pharma.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
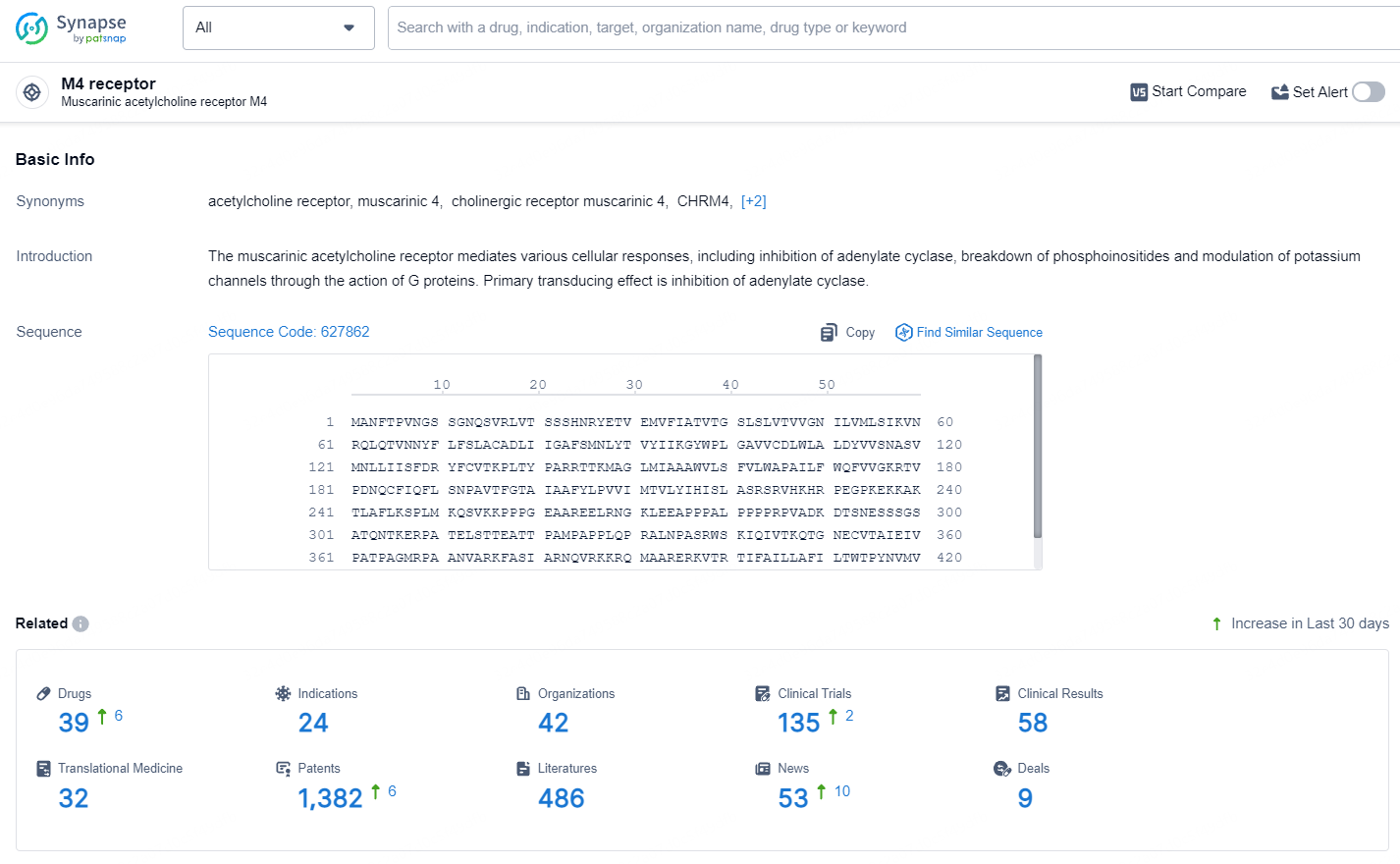
According to the data provided by the Synapse Database, As of May 11, 2024, there are 39 investigational drugs for the M4 receptor, including 24 indications, 42 R&D institutions involved, with related clinical trials reaching 135, and as many as 1382 patents.
NBI-1076986 specifically targets the M4 receptor and is primarily focused on treating nervous system diseases. The drug's active indication is movement disorders. NBI-1076986 is currently in Phase 1 of clinical development, which is the earliest stage of human testing. This phase involves evaluating the drug's safety, dosage, and potential side effects in a small group of healthy volunteers or patients. Phase 1 trials are primarily focused on assessing the drug's pharmacokinetics and pharmacodynamics.