What Does Lenvatinib Do?
Lenvatinib, marketed under the brand name Lenvima, is a multikinase inhibitor specifically targeting vascular endothelial growth factor (VEGF) receptors. Developed by Eisai Inc., a leading pharmaceutical company, Lenvatinib is classified as a targeted therapy, distinguishing it from traditional chemotherapy drugs. It has been approved for the treatment of several types of cancer, including thyroid cancer, advanced kidney cancer, endometrial cancer, and liver cancer, particularly in cases where other treatments have proven ineffective. The drug works by inhibiting the activity of protein kinases, which are enzymes that can trigger cancer cell growth and blood vessel formation, a process known as angiogenesis. By blocking these enzymes, Lenvatinib effectively starves the tumor of its blood supply, thus inhibiting its growth and spread.
Lenvatinib Mechanism of Action
Lenvatinib's mechanism of action involves the selective inhibition of multiple kinases, including those involved in angiogenesis and tumor growth. It achieves this by binding to the ATP-binding site of the kinase, thereby preventing the phosphorylation of the target protein, which is essential for its activation. This dual action on both tumor blood supply and direct cancer cell signaling makes Lenvatinib a potent therapeutic option for various malignancies.
How to Use Lenvatinib
Lenvatinib is administered orally, typically once daily at a prescribed dosage. The specific dose and duration of treatment are determined by the patient's condition, weight, and response to the medication. It is crucial to adhere to the prescribed schedule and dosage to ensure the drug's effectiveness and to minimize the risk of adverse effects. Lenvatinib can be taken with or without food, but it is important to maintain a consistent routine. If a capsule is difficult to swallow, it can be dissolved in water or apple juice as per the instructions provided. Medical supervision is required to monitor the patient's response and to manage any side effects that may arise.
Lenvatinib Side Effects
While Lenvatinib has demonstrated efficacy in treating certain cancers, it also comes with a range of potential side effects. These may include but are not limited to hypertension, diarrhea, fatigue, decreased appetite, and mouth sores. More severe side effects can involve perforations or fistulas in the gastrointestinal tract, severe chest pain, shortness of breath, and signs of heart problems. It is vital for patients to be aware of these potential side effects and to seek immediate medical attention if they occur.
Drug Interactions with Lenvatinib
The effectiveness and safety of Lenvatinib can be influenced by other medications a patient may be taking. Concomitant use of certain drugs, particularly those that affect the heart, blood pressure, or have a potential to cause infections, could increase the risk of serious heart problems. Therefore, it is essential to disclose all current medications, including prescription drugs, over-the-counter products, vitamins, and herbal supplements, to the healthcare provider. Notably, Lenvatinib may also interact with medications used to treat osteoporosis, necessitating close monitoring and possible dosage adjustments. In conclusion, Lenvatinib is a valuable addition to the arsenal against cancer, offering a targeted approach to treatment with the potential for significant patient benefit. However, its use requires careful consideration of individual patient factors, strict adherence to medical guidance, and vigilant monitoring for side effects and drug interactions. As with all medications, the balance between potential benefits and risks must be carefully weighed to ensure the best possible outcome for the patient.
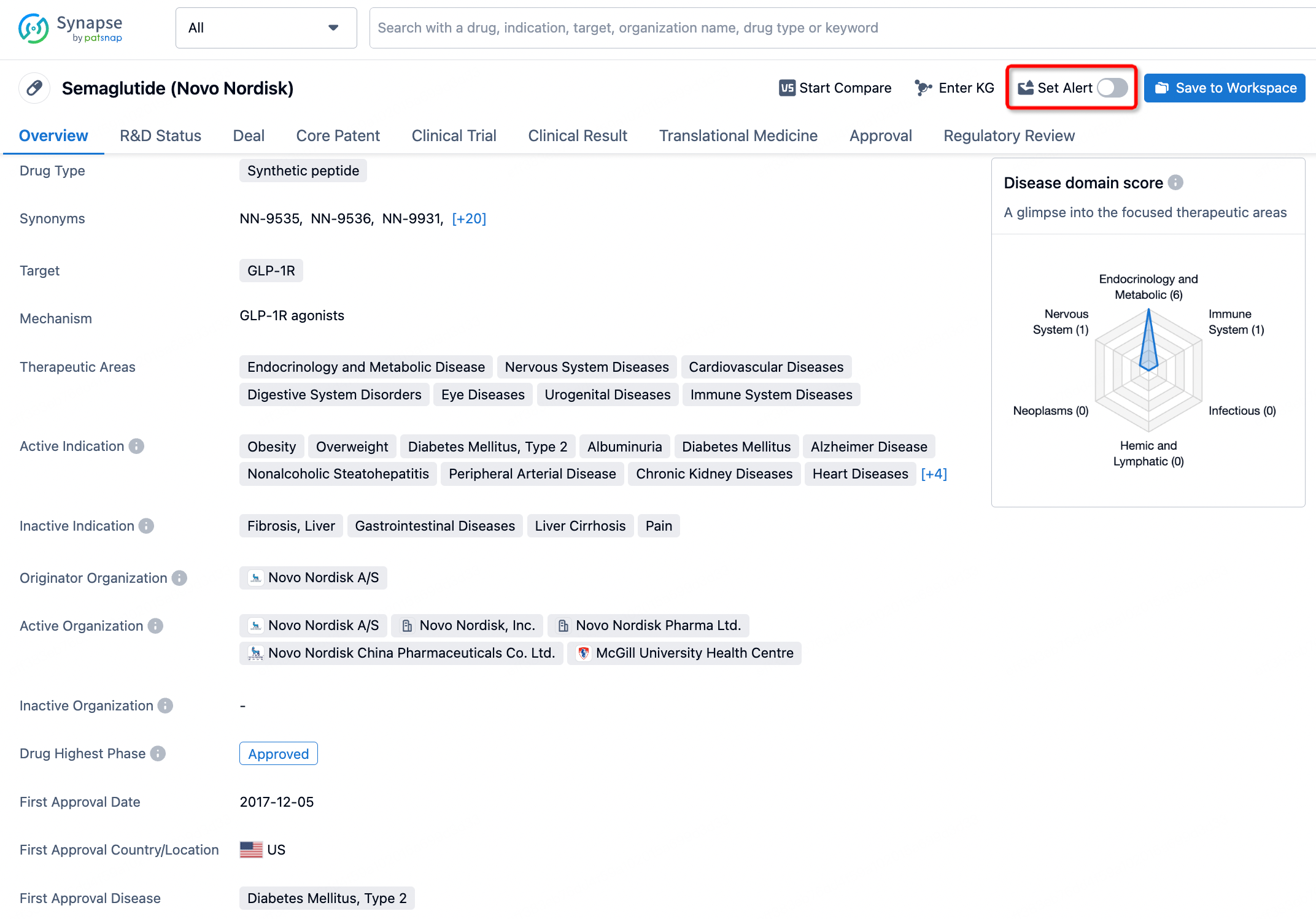
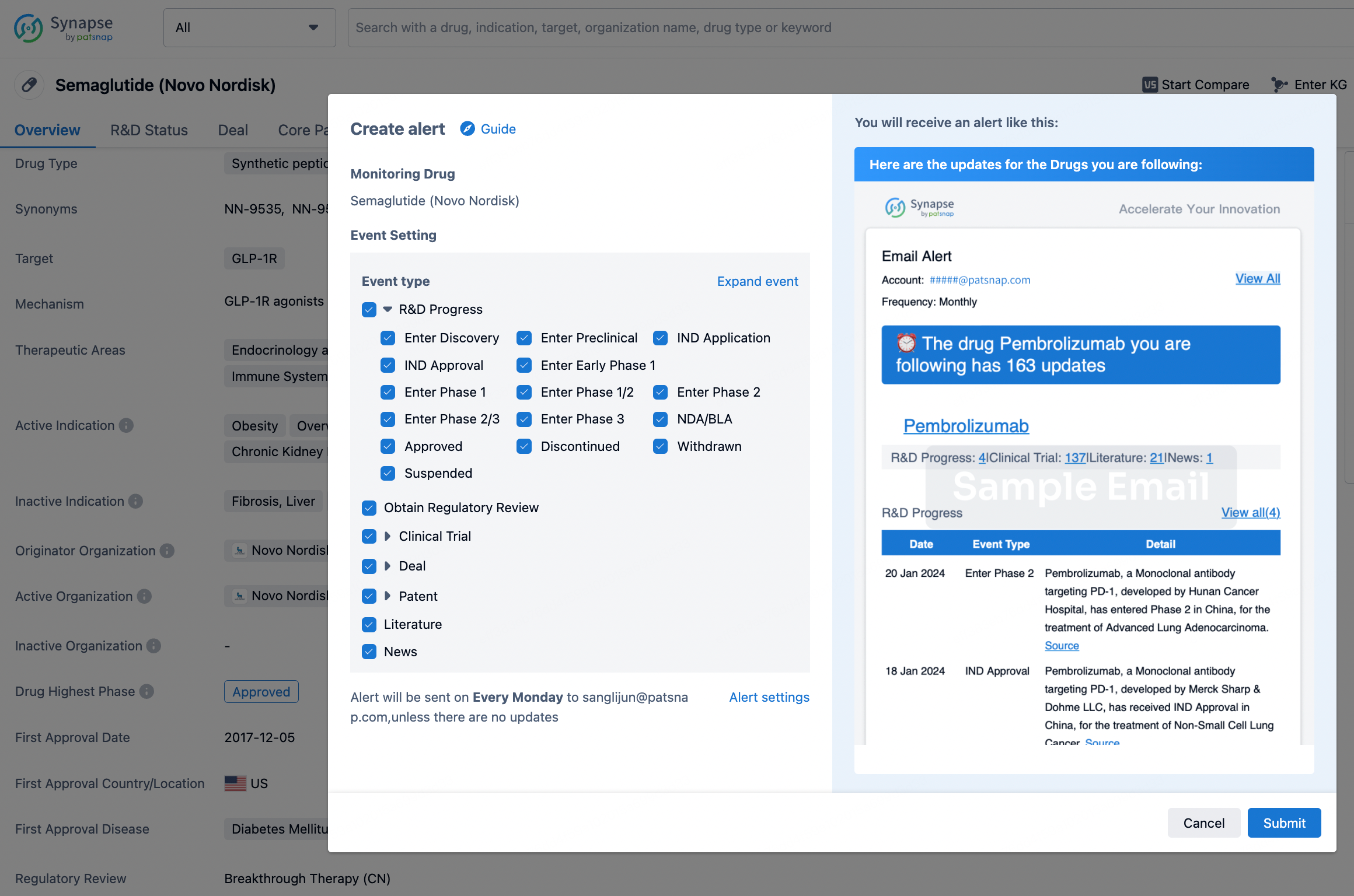
How to obtain the latest development progress of all drugs?
In the Synapse database, you can keep abreast of the latest research and development advances of all drugs anywhere and anytime, daily or weekly, through the "Set Alert" function. Click on the image below to embark on a brand new journey of drug discovery!