AstraZeneca JMC Reports on RXFP1 Agonist AZD5462
Recently, AstraZeneca reported in detail the discovery process of the RXFP1 agonist AZD5462, currently in Phase II clinical trials, in two consecutive articles published in the top-tier journal JMC (Journal of Medicinal Chemistry). In 2013, the National Institutes of Health (NIH) in the United States identified the compound ML290 from a high-throughput screen of over 350,000 compounds. Nearly a decade afterward, AstraZeneca optimized this lead compound to ultimately develop AZD5462, which became the first orally administered small molecule RXFP1 agonist to enter clinical trials in June 2022.
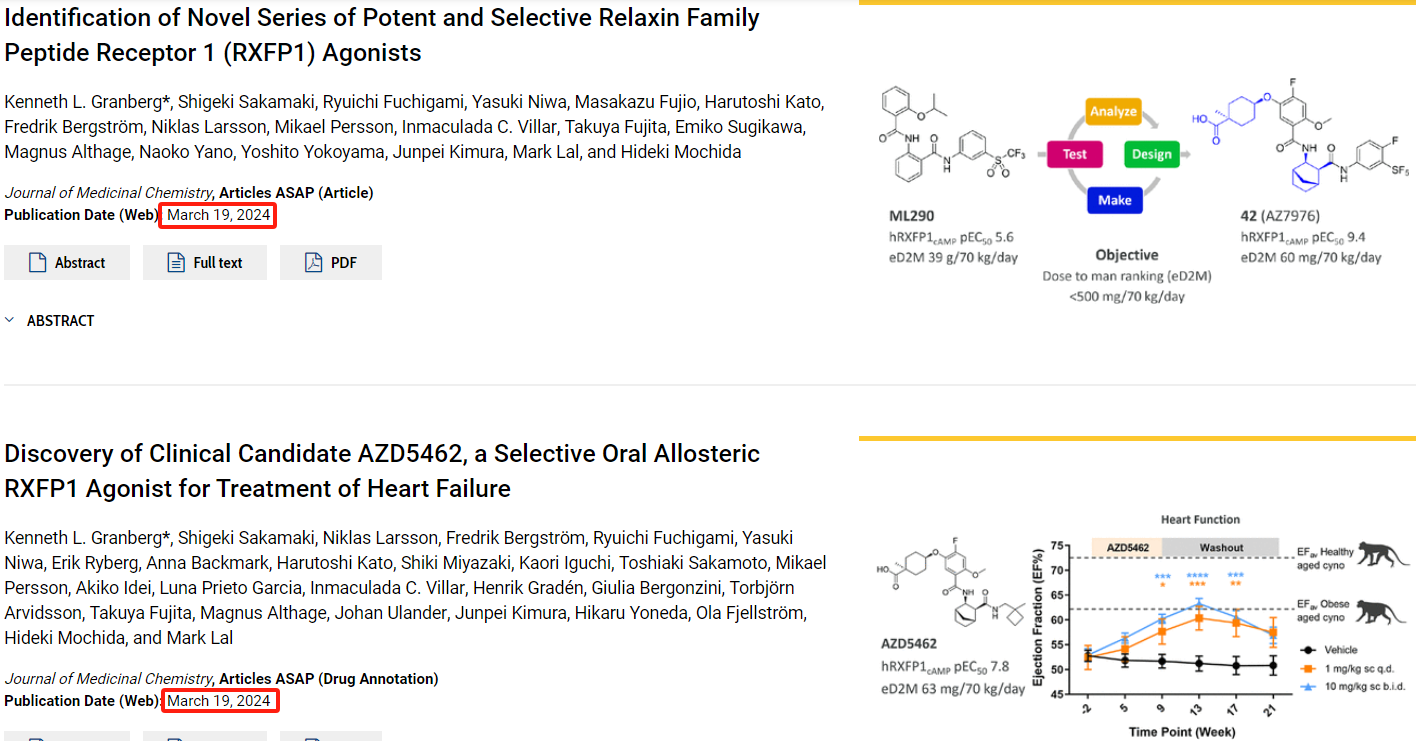
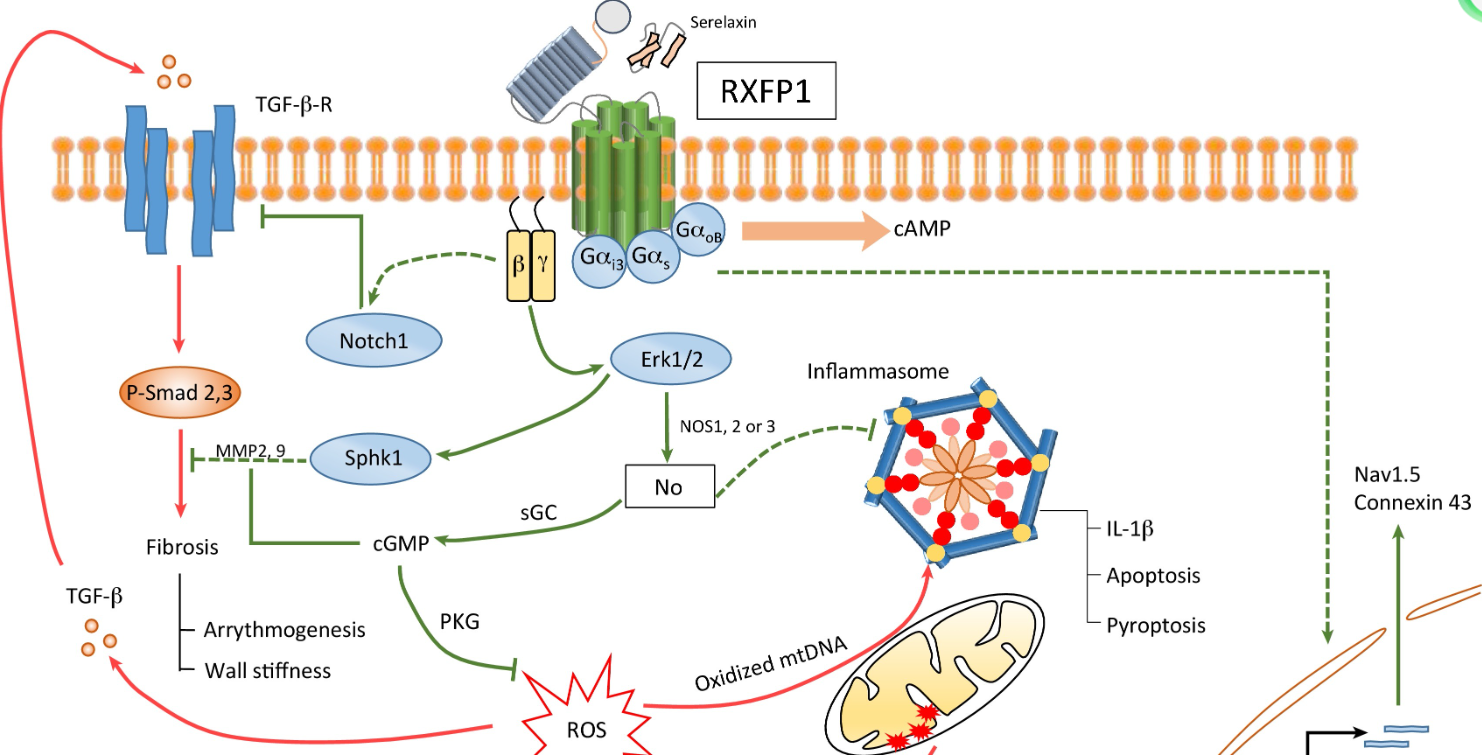
Relaxin Family Peptide Receptor 1 (RXFP1) is a receptor for relaxin-2, a key modulator of reproductive and cardiovascular physiology. RXFP1 is a thoroughly validated target and an important member of the pharmacologically relevant GPCR family 1c. Its activation by relaxin hormones is associated with hemodynamic effects, anti-fibrotic, and anti-inflammatory properties. Activation of RXFP1 can reduce vascular resistance and potentially affect pulmonary pressures, thereby increasing cardiac output and enhancing perfusion of central vascular beds in vital organs such as the kidneys, lungs, and the liver.
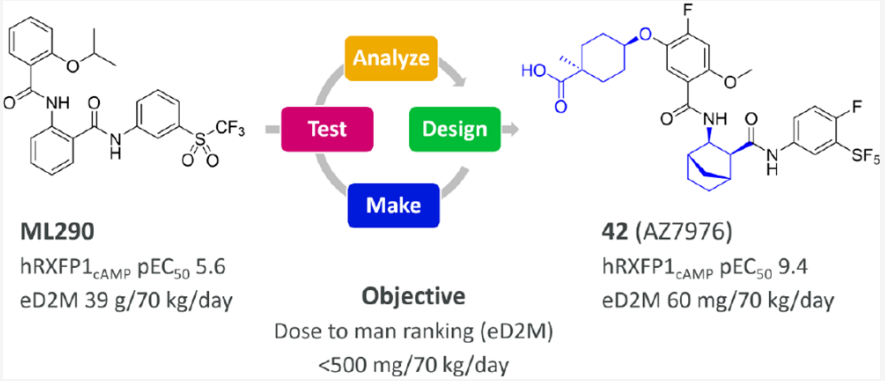
The first article by AZ in JMC primarily details the discovery process from ML290 to AZ7976. The main purpose of the modifications was to enhance the activity of hRXFP1cAMP and to improve the metabolic stability of the molecule across various species, with the goal of achieving a human dosage of less than 500 mg/70 kg/day. A key transformation was the replacement of the methylenedioxyphenyl derivative fragment with the anthranilic acid fragment in ML290. Investigating the 5-position substitution on the benzoic acid ester was crucial to the hRXFP1cAMP SAR, and introducing a carboxylic acid at the 5-position improved several properties such as solubility and LogD.
The second article mainly revolves around the transformation of AZ7976 into AZD5462. By optimizing the highly active, but metabolically unstable and poorly soluble hRXFP1 agonist AZ7976, the clinical candidate drug AZD5462 was identified. Structural changes were primarily reflected in the removal of the SF5 substituent and the substitution of the right-hand phenyl ring with an aliphatic fragment, further reducing the LogD.
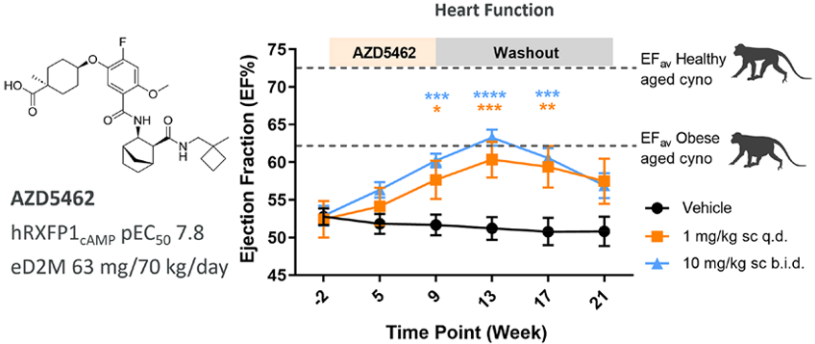
The therapeutic potential of AZD5462 was evaluated in a heart failure model of cynomolgus monkeys. After 8 weeks of treatment with AZD5462, significant improvements in functional cardiac parameters, including LVEF, were observed at weeks 9, 13, and 17, while there were no effects on heart rate or mean arterial blood pressure.
Safety is of paramount importance for RXFP1 agonists aimed at chronic conditions. AZD5462 demonstrated a balanced profile of properties, including no risk of CYP450 enzyme inhibition, no risk of TDI (CYP3A4/5), no hERG toxicity risk, and no Ames toxicity risk. In vivo experiments showed that AZD5462 was well tolerated in both rats and cynomolgus monkeys, and it has successfully completed a Phase I safety study in healthy volunteers.
These data suggest that AZD5462 is a small-molecule pharmacological analog of relaxin H2 signaling for RXFP1, with the potential to become a new treatment option for patients with heart failure.
A search in the Synapse database for AZD5462 revealed four clinical trials, of which three Phase I trials have been completed. A Phase IIb clinical trial is set to start recruiting patients on June 10, 2024. The Phase IIb trial of AZD5462, carried out in patients with stable chronic heart failure, is a two-cohort, randomized, placebo-controlled, double-blind, multicenter, dose-range study. The main objective is to assess the impact of AZD5462 on cardiac function in participants with chronic heart failure (HF).
The patent containing the compound AZD5462 is WO2022122773A1, applied for by AstraZeneca, which includes a total of 624 embodiments. In addition to AZD5462, it also provides protection for AZD3427 (recombinant peptide). The earliest priority date for patent WO2022122773A1 is December 8, 2020, and it was officially filed on December 7, 2021, and published on June 16, 2022. AstraZeneca demonstrated impressive speed, taking only a year and a half from the initial patent application to entering clinical trials.
From the 25 patent applications identified via searches for the target RXFP1 and the company AstraZeneca, one composition patent application filed on June 6, 2023, WO2023237512A, indicates that AstraZeneca likely plans to combine AZD5462 with its blockbuster drug Dapagliflozin. According to the AstraZeneca financial report for 2023, sales of Dapagliflozin approached $6 billion.
Currently, only one drug targeting RXFP1 has been marketed, which is serelaxin, a recombinant natural hormone analog developed by Novartis. Agonists in clinical development include the relaxin analog AZD3427 (Phase II), the small molecule AZD5462 (Phase II); Tectonic Therapeutic's TX-000045 is in Phase I clinical trials and plans to initiate Phase II within the year. On January 30, 2024, the publicly traded company AVROBIO announced the acquisition of the startup Tectonic Therapeutic, including this RXFP1 agonist, though the transaction amount was not disclosed; Moderna Therapeutics is developing the mRNA-based agonist mRNA-0184 (Phase I), which AstraZeneca acquired from Moderna on March 21, 2013, in a deal with an upfront payment of $240 million; BMS's BMS-986259 is currently preclinical with no progress.
More than a decade has passed since the National Institutes of Health (NIH) in the United States first reported the structure of ML290. AstraZeneca has established a broad portfolio in this area, which not only includes the recombinant peptide AZD3427 in Phase II clinical trials but also an mRNA-based agonist, mRNA-0184 (Phase I), acquired from Moderna, and the oral small molecule AZD5462. With AZD5462 and TX-000045 nearing the commencement of Phase II patient recruitment to evaluate efficacy, enthusiasm for research and development in this field is set to reignite. We look forward to the announcement of more positive results, bringing hope to patients with cardiovascular diseases.
Reference
1.Ram W. Sabnis. Novel RXFP1Modulators for Treating Heart Failure. ACS Med. Chem. Lett. 2022, 13, 1213−1214.
2.Maik Gollasch et al; RXFP1 Receptor Activation by Relaxin-2 Induces Vascular Relaxation in Mice via a Gαi2-Protein/PI3Kß/γ/Nitric Oxide-Coupled Pathway. doi: 10.3389/fphys.2018.01234.
3.Pamela Brown et al; Recent trends in medicinal chemistry and enabling technologies. Highlights from the Society for Medicines Research Conference. Drugs of the Future 2023, 48(3): 211-219.