Vertex Progresses VX-147 to Phase 3 Segment of Combined Phase 2/3 Study for APOL1-Related Kidney Conditions
Vertex Pharmaceuticals Incorporated revealed the progression of inaxaplin (VX-147) to the critical Phase 3 segment of the worldwide Phase 2/3 clinical study focusing on APOL1-related renal conditions. The next stage of research will examine the effects of administering a 45 mg oral dose of the drug once daily against a control group receiving a placebo, both alongside the established standard treatments.
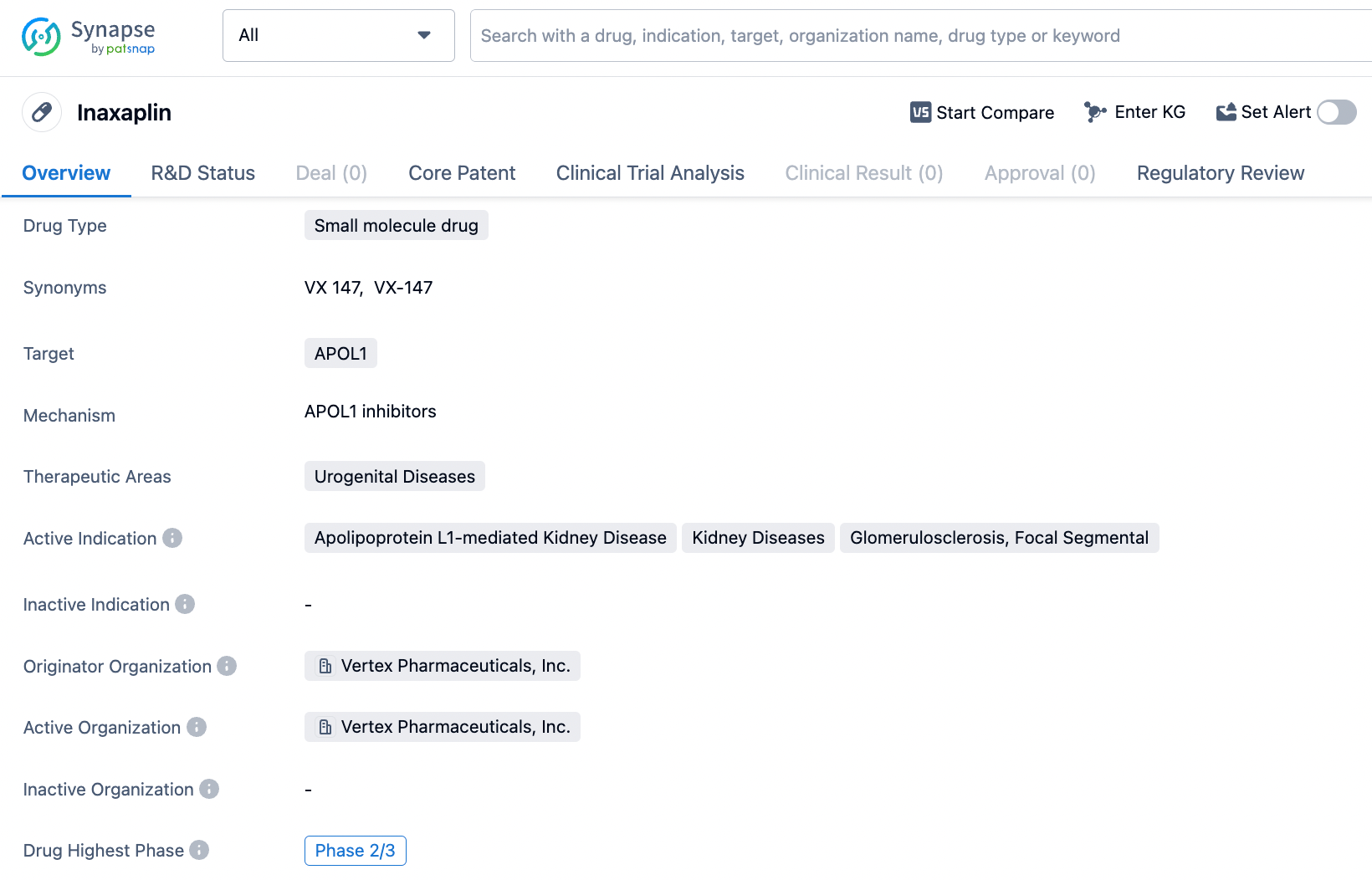
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The study aims to evaluate the effects of the drug inaxaplin on renal functionality and the presence of excessive protein in urine (proteinuria), particularly in individuals affected by a type of kidney disease called AMKD, linked to mutations in the APOL1 gene. Moreover, the research scope now encompasses young AMKD patients between 10 and 17 years of age.
Prior Phase 2a exploratory data revealed that treatment with inaxaplin for 13 weeks resulted in a significant and meaningful average decrease of 47.6% in the ratio of protein to creatinine in urine from the starting point, suggesting that inaxaplin, an orally administered APOL1 inhibitor, could effectively reduce proteinuria among AMKD sufferers.
Carmen Bozic, M.D., Vertex's EVP of Global Medicines Development and Medical Affairs and CMO, remarked, "The breakthrough achieved with inaxaplin, which targets the APOL1-mediated kidney disease at its root, was remarkable during the initial proof-of-concept study. Progressing to a Phase 3 study while also including a younger demographic represents a major leap in making this innovative treatment accessible to patients in need."
"AMKD typically escalates quickly and often goes undetected until it's considerably advanced. Currently, no therapies tailored exclusively to this condition have received approval. Inaxaplin promises to revolutionize the management of AMKD and substantively enhance patient outcomes," commented Glenn M. Chertow, M.D., M.P.H., Stanford's Professor of Medicine and head of Vertex’s APOL1 Program Steering Committee.
In recognition of its potential to address an unmet medical need, the U.S. Food and Drug Administration has provided inaxaplin with a Rare Pediatric Disease Designation and a Breakthrough Therapy Designation for treating APOL1-mediated focal segmental glomerulosclerosis. The European Medicines Agency has followed suit, assigning Priority Medicines and Orphan Drug status to inaxaplin for its use in AMKD.
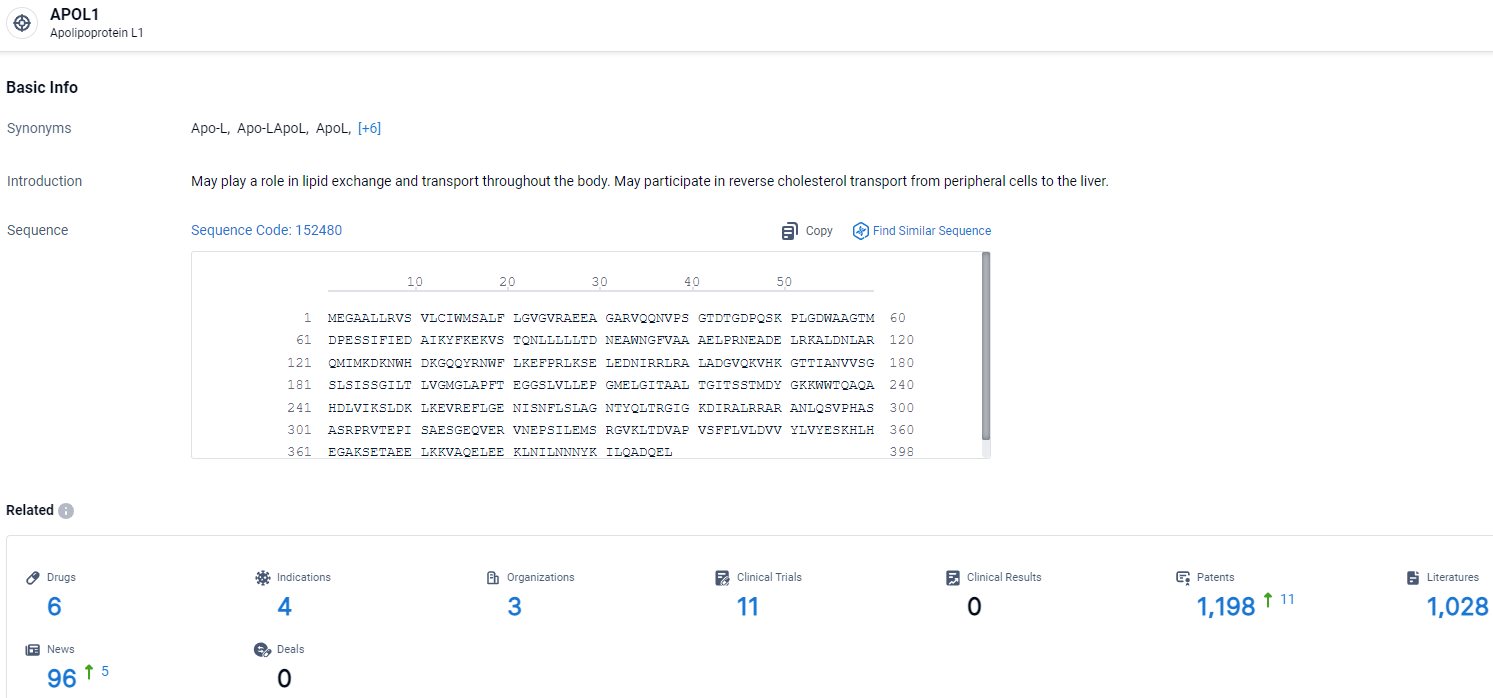
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of April 3, 2024, there are 6 investigational drugs for the APOL1 target, including 4 indications, 3 R&D institutions involved, with related clinical trials reaching 11, and as many as 1198 patents.
Inaxaplin targets APOL1 and is being evaluated for its potential in treating urogenital diseases, specifically Apolipoprotein L1-mediated Kidney Disease, Kidney Diseases, and Glomerulosclerosis, Focal Segmental. The drug is currently in Phase 2/3 of development and has received regulatory designations such as PRIME, Orphan Drug, and Breakthrough Therapy, indicating its potential importance in addressing unmet medical needs and providing improved treatment options for patients.