At SITC 2023, ENB Therapeutics presented early results from phase one of its ENBOLDEN-101 trial for platinum-resistant ovarian cancer
ENB Therapeutics, Inc., a biopharmaceutical corporation that specializes in cancer and immunity-related fields, has shared preliminary outcomes from the combinatory branch of their Phase 1b ENBOLDEN-101 research. The study was aimed at assessing the effect of combining ENB-003 with Merck's KEYTRUDA® (pembrolizumab) - an anti-PD-1 therapy, on patients dealing with progressive treatment-resistant cancers.
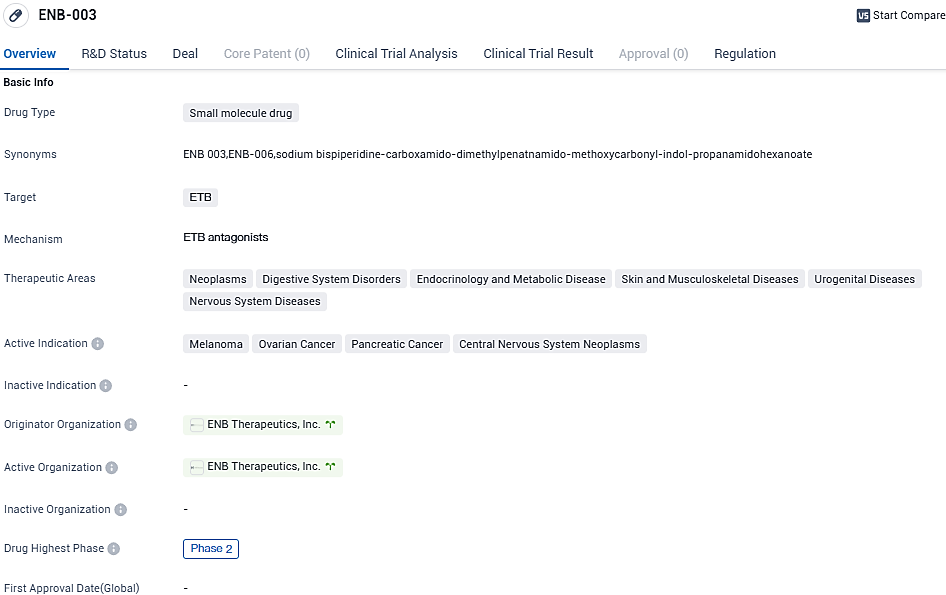
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The findings indicate promising objective responses, successful control of the condition, and increased duration of survival without disease advancement in patients with platinum-resistant/refractory ovarian cancer. ENB-003, an ETBR antagonist, when combined with pembrolizumab, seems to yield promising results for microsatellite stable, platinum-resistant/refractory ovarian cancer.
Information from the ENBOLDEN-101 Phase 1B investigation was shared on November 3, 2023, in a poster session during the SITC 2023 conference, which took place from November 1-5 in San Diego, California, USA.
The study included five patients with advanced microsatellite stable PROC who had experienced disease advancement after at least one previous treatment. The initial phase of treatment, a single week of ENB-003 monotherapy, was followed by repetitive three-week cycles of ENB-003 in tandem with KEYTRUDA.
The findings revealed the treatment approach was safe and well received. In terms of MSS PROC, the objective response rate and control rate were 40% and 80%, respectively. This includes two patients with partial responses reflecting a 95% and 33% decrease in tumor mass, respectively, and four patients whose disease status remained stable.
A total of 80% PROC patients exhibited overall shrinking of the target lesions. This is remarkably better than the historical data indicating ~20% survival without disease progression at six months for single agent anti-PD1, and an ~8% ORR and ~22% DCR.
“These findings verify the safety of the combination of ENB-003 and KEYTRUDA, and show promising indications of clinical activity, strengthening the possibility of the combination as an impactful immunotherapy strategy for primary MSS PROC - a disease that has shown very little response to current therapies." said Sumayah Jamal, MD-Ph.D., President, Chief Scientific Officer, and Co-Founder of ENB Therapeutics.
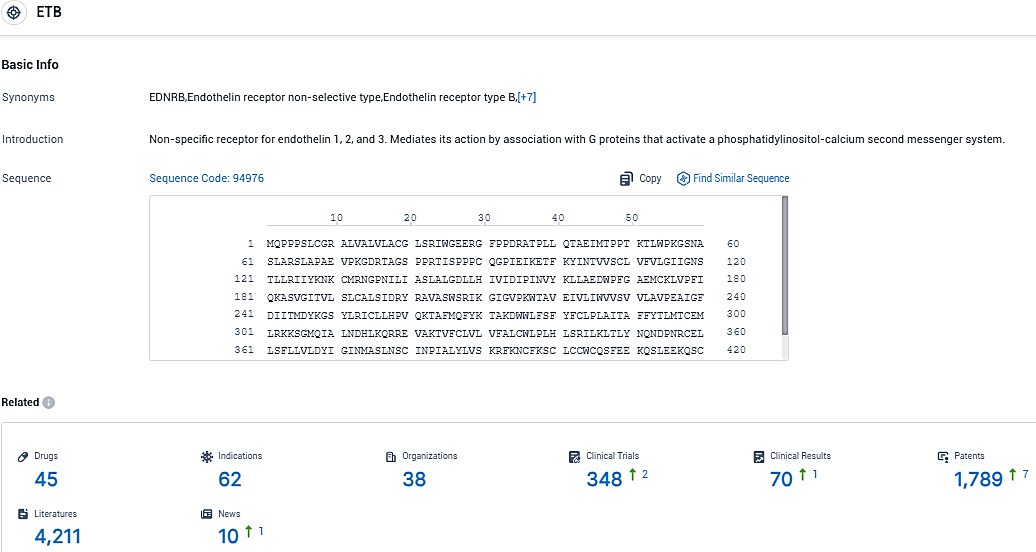
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 14, 2023, there are 45 investigational drugs for the ETB target, including 62 indications, 38 R&D institutions involved, with related clinical trials reaching 348, and as many as 4211 patents.
ENB-003 is a selective endothelin B receptor (ETBR) inhibitor that, in preclinical studies, enhances efficacy of CAR-T and anti-PD-1 in solid tumors across multiple cancer types in preclinical studies. The Phase 2 portion of the ENB-003 + pembrolizumab combination study is expected to start in the first half of 2024. The trial will enroll MSS primary PROC, as well as MSS pancreatic cancer patients and patients with other advanced solid tumors that have failed standard of care.