At the 2023 SNO Conference, Imvax showcased new data supporting its main project, IGV-001, for newly discovered Glioblastoma
Imvax, Inc., a company in the clinical-phase biotech sector specializing in the creation of individualized immunotherapies derived from entire tumors, has disclosed information about its duo of poster exhibitions at the 28th yearly gathering of the Society for NeuroOncology. This conference took place in Vancouver, British Columbia, Canada, spanning from November 15-19, 2023.
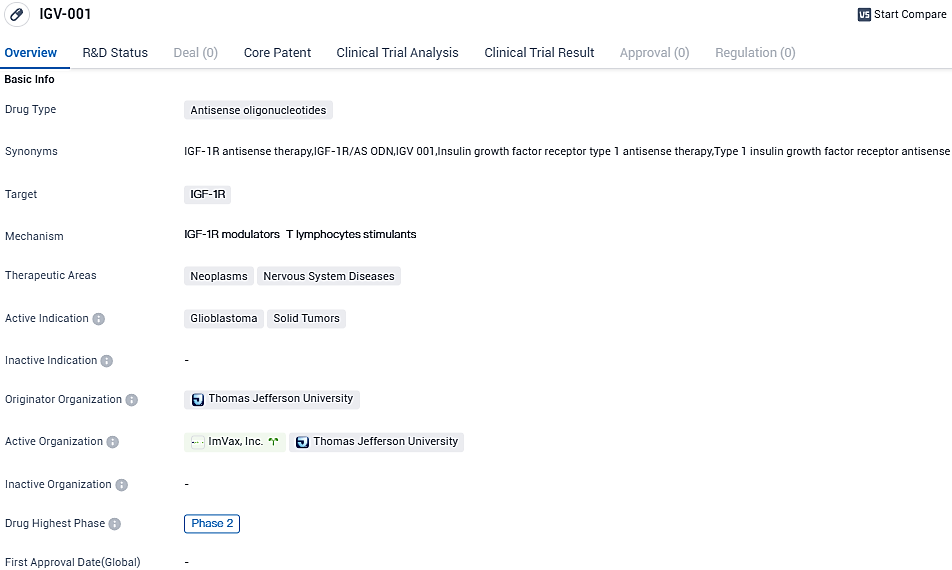
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
During the conference, Imvax brought forward fresh results from their Phase 1b trial of IGV-001 involving patients recently diagnosed with glioblastoma. Further data related to Imvax's currently active, multicenter, double-blind, placebo-controlled Phase 2b clinical trial of IGV-001 for patients with ndGBM was shared by Ian Y. Lee, M.D., a Neurosurgeon from the Henry Ford Health System.
"The importance these presentations attach to the Phase 1b findings for the IGV-001 project, reflecting the scientific rigour implemented in the ongoing Phase 2b trial, is impressive" stated David W. Andrews, M.D., the Chief Medical Officer at Imvax.
"I'm happy to note that the Phase 2b study is seeing successful enrollment and the process is expected to be concluded by the first half of 2024." Titled "Additional outcomes from a Phase 1b trial of IGV-001 in patients recently diagnosed with glioblastoma," the initial poster presentation emphasized a meaningful correlation in Imvax's Phase 1b trial of IGV-001, between PFS and overall survival of intent-to-treat study participants. This implies that median PFS could be used as an endpoint in forthcoming ndGBM clinical trials.
The subsequent poster presentation, named “A multicenter, double-blind, Phase 2b study of IGV-001, an autologous cell immunotherapy with antisense oligo IMV-001 targetting IGF-1R, as opposed to placebo, in patients recently diagnosed with glioblastoma,” gave details about the ongoing Phase 2b trial, including its goals, design, endpoints, locations, and significant criteria for inclusion and exclusion.
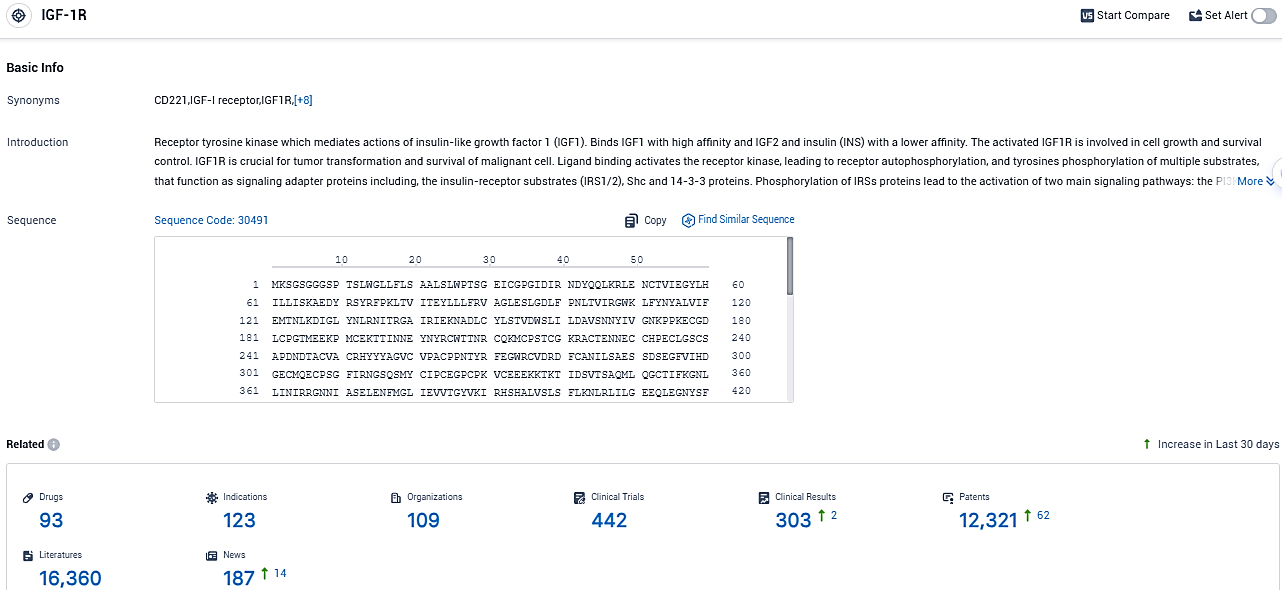
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 28, 2023, there are 93 investigational drugs for the IGF-1R target, including 123 indications, 109 R&D institutions involved, with related clinical trials reaching 442, and as many as 12321 patents.
IGV-001 is an antisense oligonucleotide drug that targets the IGF-1R and is being developed for the treatment of neoplasms and nervous system diseases, specifically glioblastoma and solid tumors. It is currently in Phase 2 of clinical development, indicating that it has shown promise in early-stage trials.