Atamyo Therapeutics, known as ATA-200, designed to treat Limb-Girdle Muscular Dystrophy Type 2C/R5, achieves significant progress
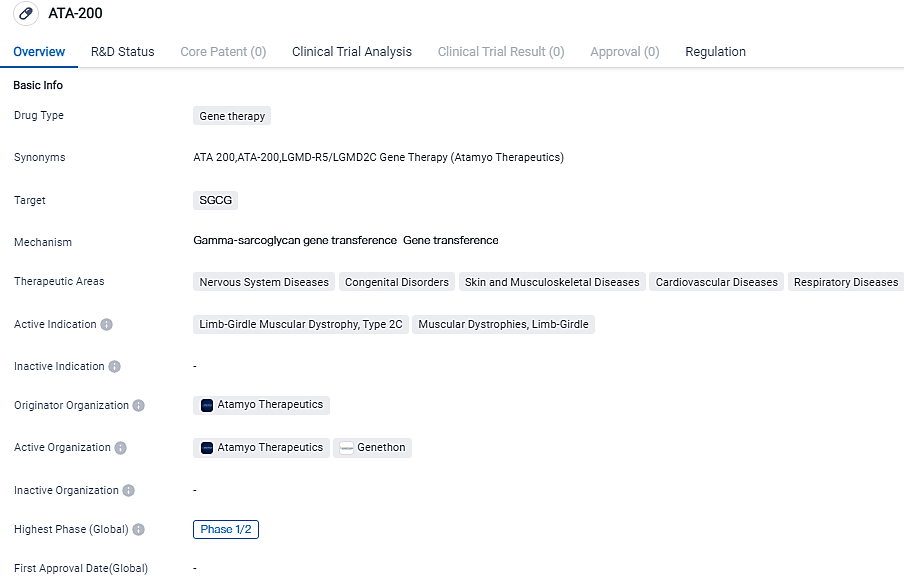
Atamyo Therapeutics, a biotech firm focused on crafting next-generation gene treatments targeting at neuromuscular conditions, made public its submission of a CTA for ATA-200 in European territories. ATA-200 is their gene treatment designed for addressing limb-girdle muscular dystrophy Type 2C/R5 associated with γ-sarcoglycan (SGCG).
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The firm also disclosed that ATA-200 has been granted a non-dilutive financial support amounting to €8m from France 2030 public scheme administered by Bpifrance, aimed at backing its clinical experiments and manufacturing development plans.
This clinical research is characterized as a multicenter, Phase 1b, open-label, dose escalation investigation that assesses safety, pharmacodynamic, effectiveness, and immunogenicity of intravenous ATA-200, which is a single-dose Adeno-Associated Virus vector holding the human γ-sarcoglycan transgene.
"LGMD-R5 emerges as the most swiftly advancing and debilitating variant of LGMDs, with symptom onset during early childhood, loss of mobility prior to adulthood, and common cardiac dysfunction. This disease currently lacks a cure", remarked Dr Sophie Olivier, the Chief Medical Officer of Atamyo.
"ATA-200 includes a novel promoter that increases the safety of gene therapy for liver and heart," stated Isabelle Richard, Ph.D., Chief Scientific Officer and Co-founder of Atamyo. "This pioneering experimental therapy signifies a fresh beacon of hope for patients."
"We are quite excited to lodge this European Clinical trial and to obtain such substantial public funding for the severely detrimental LGMD2C/R5 illness", expressed Stephane Degove, CEO and Co-Founder of Atamyo. “Our ongoing clinical trial in LGMD-R9, coupled with the introduction of the clinical scheme for LGMD-R5/2C validates our unique expertise in delivering patients afflicted by limb-girdle muscular dystrophies with an innovative range of secure and effective gene therapies." 👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
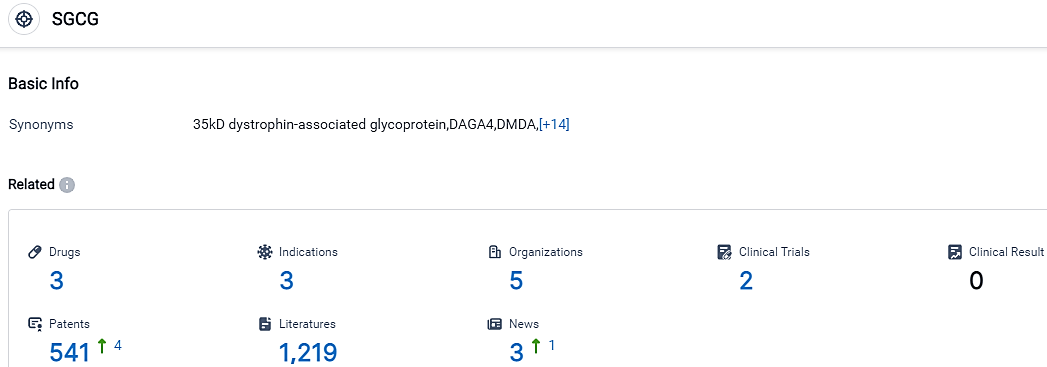
According to the data provided by the Synapse Database, As of September 22, 2023, there are 3 investigational drugs for the SGCG target, including 3 indications,5 R&D institutions involved, with related clinical trials reaching 2,and as many as 541 patents.
ATA-200 aims to address Limb-Girdle Muscular Dystrophy, Type 2C, a specific subtype of muscular dystrophies. Further research and development are necessary to determine the safety and efficacy of ATA-200 in treating Limb-Girdle Muscular Dystrophy, Type 2C. Atamyo plans to initiate dosing in patients for ATA-200 in the first half of 2024.