Endeavor BioMedicines Secures $132.5M in Oversubscribed Series C to Advance Medical Pipeline
Endeavor BioMedicines, Inc., a biotechnology firm in the clinical phase developing drugs to potentially transform the treatment of life-threatening diseases, has announced the completion of its Series C financing round, amassing $132.5 million, with $5 million of this total coming from the conversion of a convertible instrument.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The recent funding round, which was notably oversubscribed, was spearheaded by AyurMaya, a subsidiary of Matrix Capital Management. Additional new investors included Fidelity Management & Research Company, Invus, SymBiosis, Velosity Capital, and Woodline Partners. Noteworthy continued backing was also provided by existing investors such as those funds managed by abrdn Inc., Ally Bridge Group, Avidity Partners, Eckuity Capital, Longitude Capital, Omega Funds, Perceptive Advisors, Piper Heartland Healthcare Capital, Silver Arch Bio, and T. Rowe Price Associates.
Endeavor will allocate the capital raised towards the accelerated clinical development of ENV-101, their primary drug candidate aimed at addressing idiopathic pulmonary fibrosis and progressive pulmonary fibrosis, and the progression of ENV-501. ENV-501, a human epidermal growth factor 3 antibody-drug conjugate, is being developed to target HER3-positive solid tumors up to the clinical proof-of-concept stage.
John Hood, Ph.D., Co-Founder, CEO and Chairman at Endeavor, expressed gratitude for the robust backing of these distinguished life sciences investors. He acknowledged the significant advancements made since their early funding stages and the potential of their drug candidates to transform treatment paradigms for severe, unyielding illnesses. He emphasized the strategic alignment and strong momentum that Endeavor BioMedicines possesses, paired with a capable team and adequate funding to propel their mission of delivering groundbreaking therapies to those in urgent need.
Key insights derived from the latest completed Phase 2a clinical trial, which was a randomized, double-blind, placebo-controlled study of ENV-101, revealed the drug's capability to potentially alter disease course significantly and improve treatment efficacy for patients suffering from idiopathic pulmonary fibrosis. These findings are scheduled to be disclosed at the American Thoracic Society International Conference this coming May. Additionally, Endeavor is planning to launch a Phase 2b trial targeting IPF patients and a separate cohort for those with progressive pulmonary fibrosis in 2024.
Karan Takhar, Senior Managing Director at Matrix, praised Endeavor BioMedicines’ groundbreaking drug candidates for their potential to fundamentally challenge the causative processes of severe and life-threatening conditions. As a new board member following the Series C financing, Takhar anticipates working closely with the executive team and board members at Endeavor to aid in steering the company towards achieving significant clinical milestones in 2024 and beyond.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
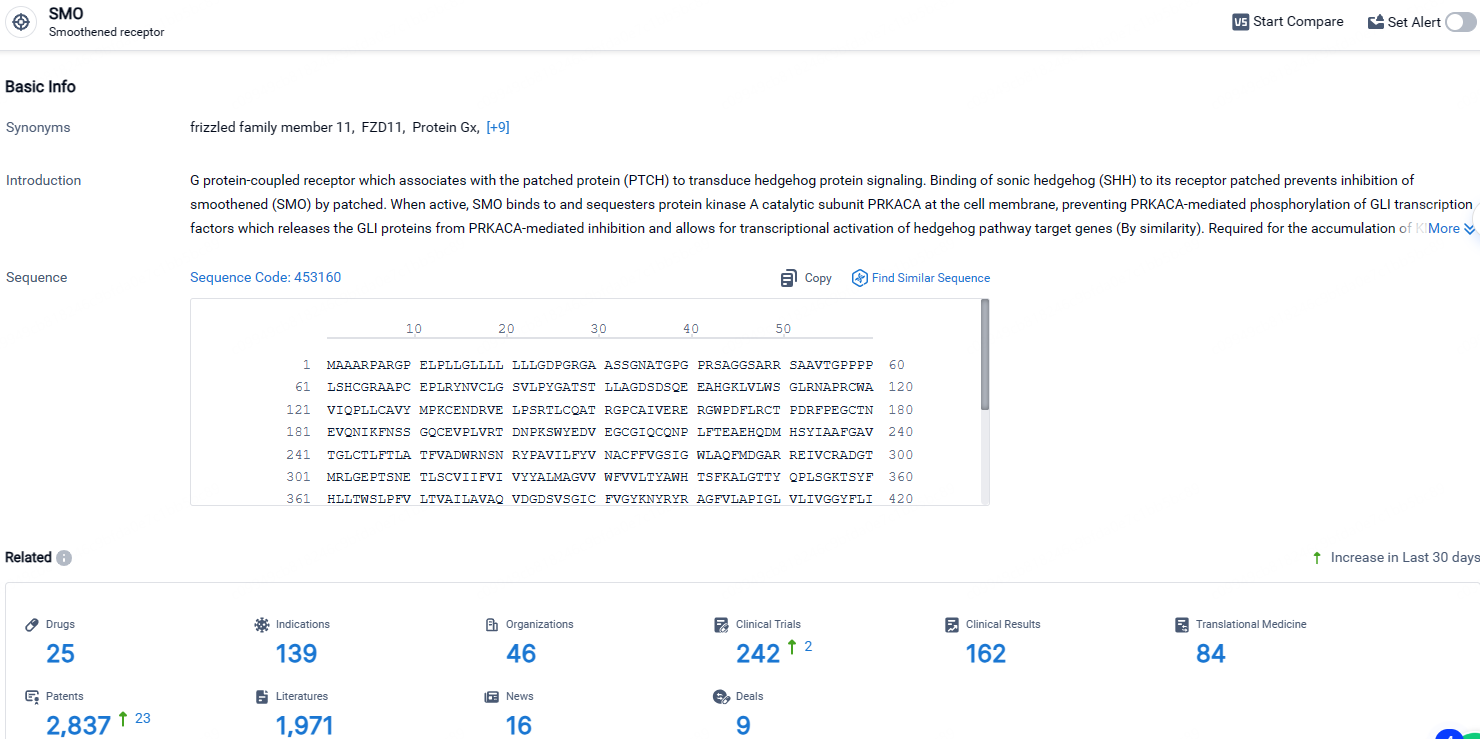
According to the data provided by the Synapse Database, As of April 28, 2024, there are 25 investigational drugs for the SMO targets, including 139 indications, 46 R&D institutions involved, with related clinical trials reaching 242, and as many as 2837 patents.
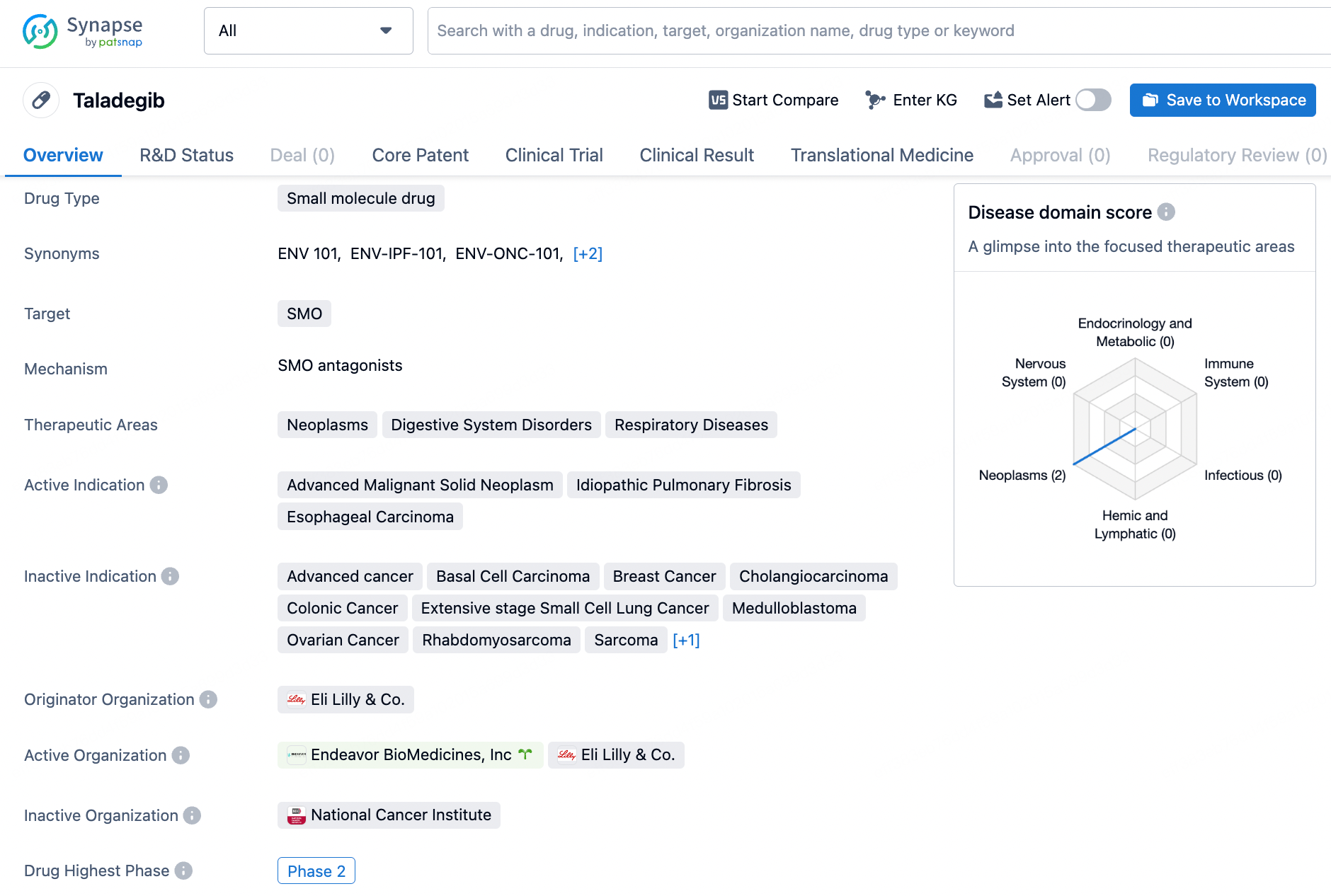
Taladegib is a small molecule drug that targets the SMO protein. It has shown promise in treating neoplasms, digestive system disorders, and respiratory diseases. The drug is actively indicated for advanced malignant solid neoplasms, idiopathic pulmonary fibrosis, and esophageal carcinoma. Taladegib has reached Phase 2 of clinical trials, signifying its progress in the drug development process.