Aulos Bioscience Shares Favorable Preliminary Phase 1/2 Data for AU-007 at 2024 ASCO Meeting
Aulos Bioscience, an immuno-oncology firm aiming to transform cancer treatment with leading IL-2 therapies, revealed preliminary outcomes from its Phase 1/2 study of AU-007. This is the inaugural human monoclonal antibody crafted with artificial intelligence to undergo clinical testing. The findings are set to be showcased in a poster session at the American Society of Clinical Oncology 2024 Annual Meeting in Chicago, Illinois.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"The extent and breadth of anti-tumor effects observed across various solid tumors are truly impressive at this early stage of clinical development, especially the partial responses and complete metabolic responses. The observed durability of tumor shrinkage in several patients indicates the potential formation of immune memory against the cancer cells. These findings support our long-standing hypothesis that AU-007 could expand the therapeutic window of IL-2 by steering IL-2 towards CD8+ T cells and natural killer cells, which are effective in tumor destruction, and away from immunosuppressive regulatory T cells and the endothelial system," stated Aron Knickerbocker, CEO of Aulos Bioscience.
"Unlike other IL-2 therapies in development, which focus on modifying IL-2 itself, AU-007 is a human antibody, a well-proven therapeutic approach. This offers unique advantages and distinctions. As we begin treating patients earlier in their therapy cycles, we are confident AU-007 is on its way to being a potential best-in-class treatment option," added Knickerbocker.
Reductions in peripheral regulatory T cells and increased CD8/Treg ratios highlight AU-007’s capacity to redirect IL-2 and avoid the suppressive feedback loop to Tregs. Additionally, AU-007 and low-dose aldesleukin significantly boost peripheral natural killer cells.
Phase 2 expansion cohorts of the AU-007 trial continue patient enrollment, focusing on melanoma and RCC. The company expects to present updated clinical data in the latter half of 2024. Further Phase 2 expansion cohorts are planned to assess the combination of AU-007 and aldesleukin in second-line PD-L1+ NSCLC, both with and without the PD-L1 antibody avelumab.
AU-007 is a human IgG1 monoclonal antibody designed using artificial intelligence, specifically targeting the CD25-binding region of IL-2. Its unique mechanism of action differentiates it from other IL-2 therapies in development, leveraging IL-2 to enhance anti-tumor immune responses. This is accomplished by preventing IL-2, whether externally administered or produced by effector T cells, from binding to trimeric receptors on regulatory T cells while still allowing IL-2 to bind and expand effector T cells and NK cells.
This mechanism avoids the immune suppressive feedback loop seen with other IL-2-based treatments and promotes immune activation rather than suppression. Additionally, AU-007 stops IL-2 from binding to CD25-containing receptors on eosinophils and the vascular and pulmonary endothelium, thus potentially reducing the vascular leak syndrome and pulmonary edema associated with high-dose IL-2 therapy."
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
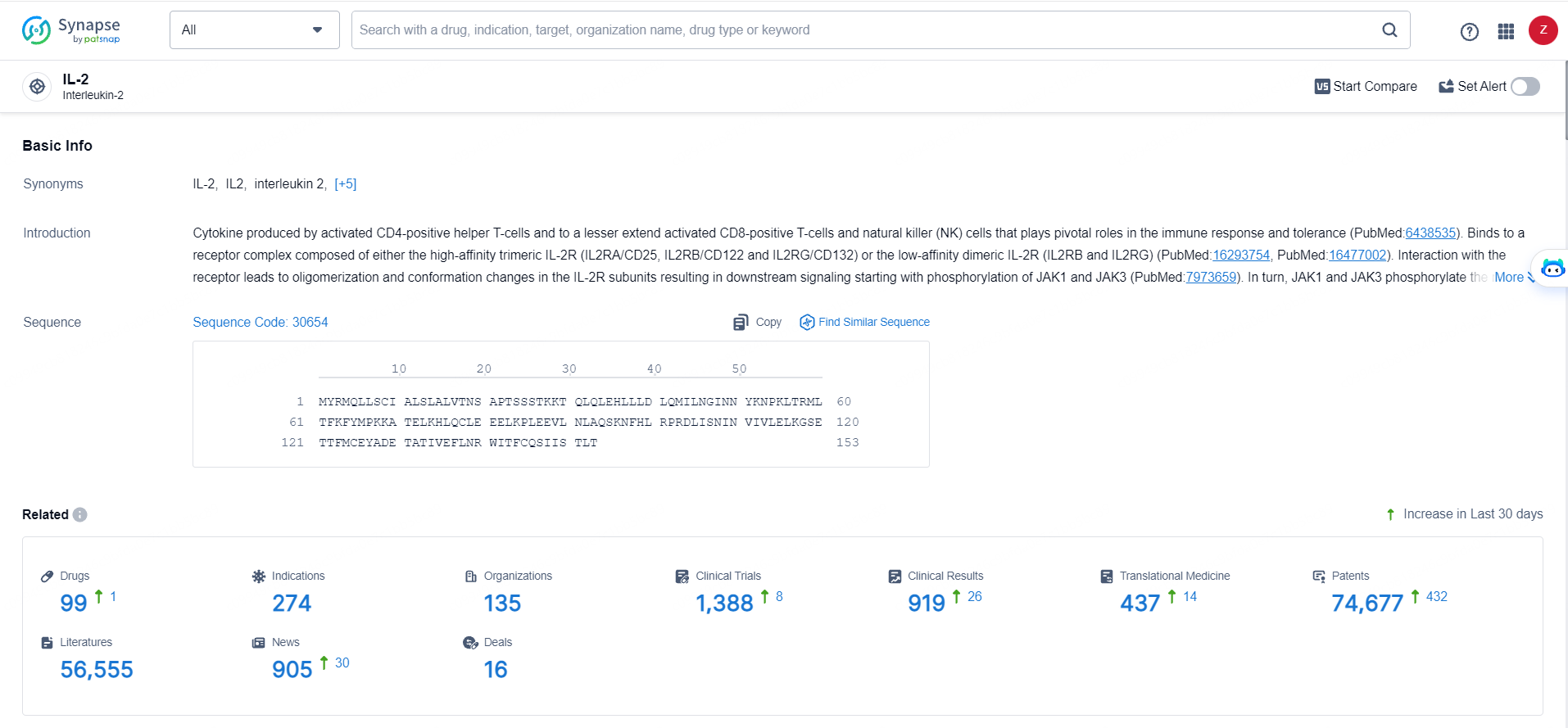
According to the data provided by the Synapse Database, As of May 28, 2024, there are 99 investigational drugs for the IL-2 targets, including 274 indications, 135 R&D institutions involved, with related clinical trials reaching 1388, and as many as 74677 patents.
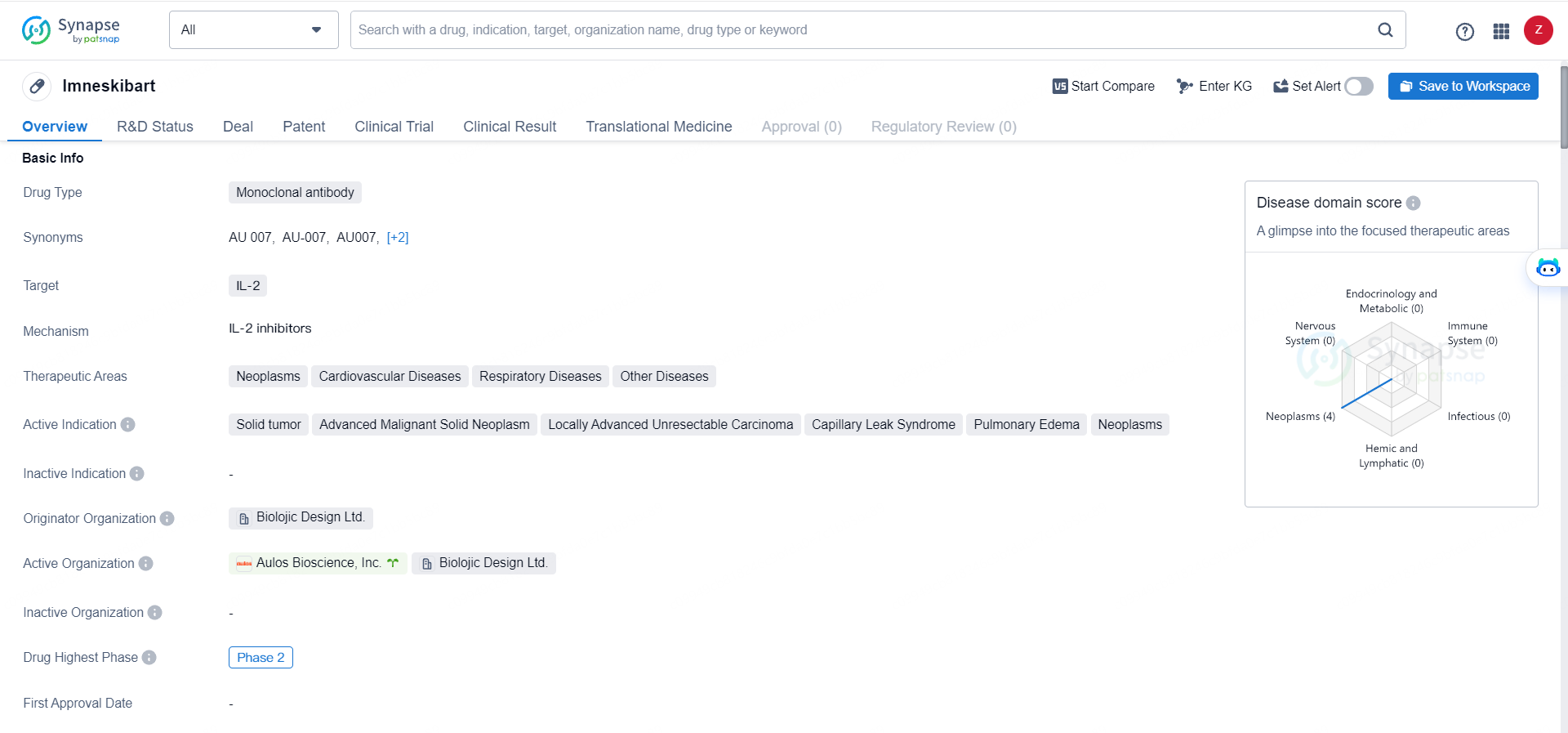
Imneskibart represents a novel approach to targeting IL-2 with a monoclonal antibody, and its advancement to Phase 2 development indicates potential for addressing a range of diseases, particularly in the field of oncology and immunology. Further clinical trials and research will be necessary to fully evaluate the efficacy and safety of Imneskibart in the treatment of neoplasms, cardiovascular diseases, respiratory diseases, and other related conditions.