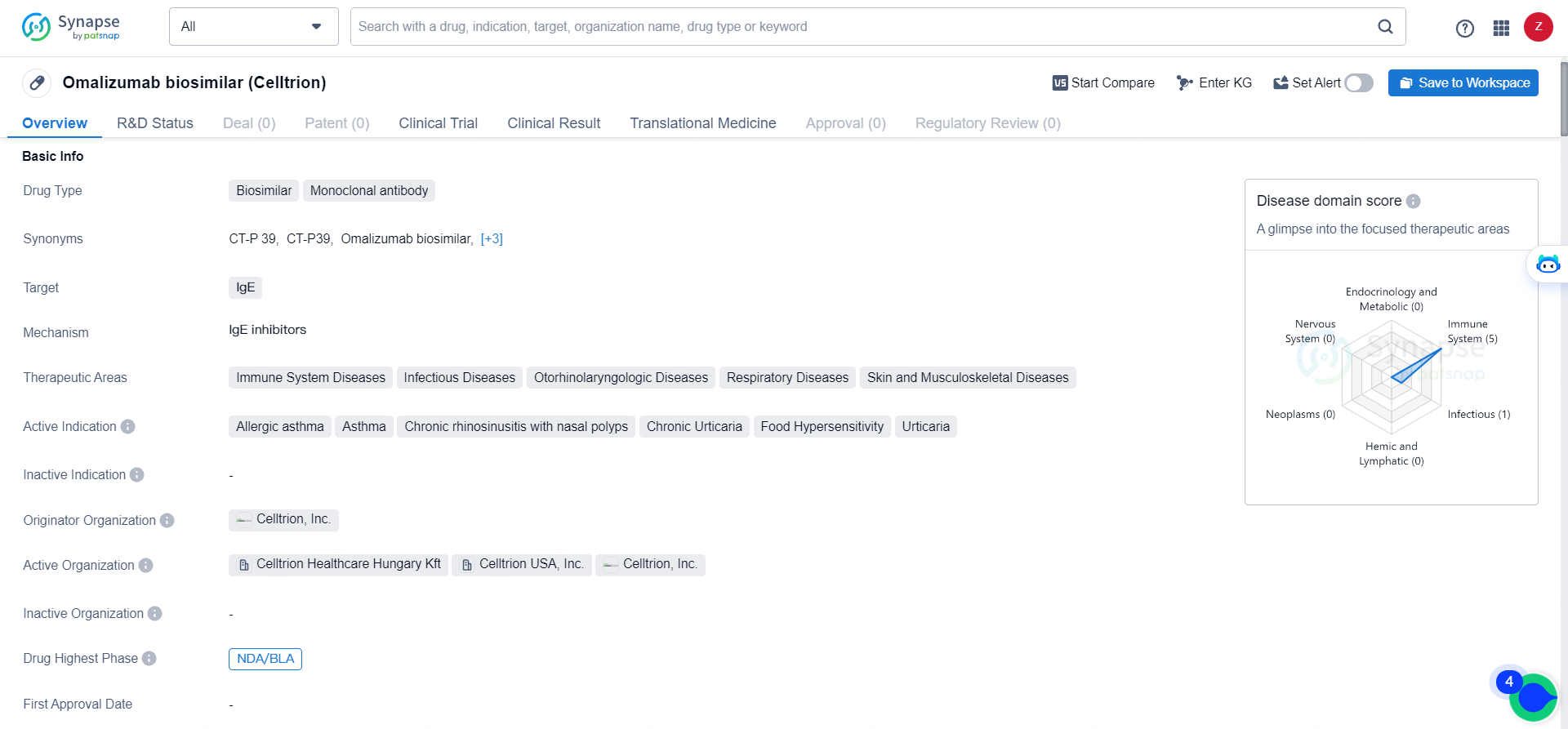
European Commission Approves Celltrion's Omlyclo®, First Omalizumab Biosimilar in Europe
Celltrion revealed that Omlyclo® (CT-P39), a biosimilar of omalizumab modeled after Xolair®, has received approval from the European Commission. The approved uses for Omlyclo® include managing allergic asthma, chronic spontaneous urticaria, and chronic rhinosinusitis with nasal polyps.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The European Commission's (EC) approval of Omlyclo® is in line with the marketing authorisation suggested by the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) in March 2024. This decision was grounded in clinical data, encompassing results from a global Phase III clinical trial aimed at assessing the efficacy, safety, and pharmacokinetics of Omlyclo® in comparison to the reference product Xolair® for patients with Chronic Spontaneous Urticaria (CSU) up to Week 40.
"For over twenty years, omalizumab, a groundbreaking monoclonal antibody targeting IgE, has transformed the management of chronic immune-mediated inflammatory diseases," noted Professor Marcus Maurer, Professor of Dermatology and Allergy, Co-Director for the Fraunhofer Site for Immunology and Allergology at the Fraunhofer Institute for Translational Medicine and Pharmacology ITMP, and Executive Director for the Institute of Allergology at Charité – Universitätsmedizin Berlin, Germany.
"Conditions such as asthma can profoundly affect patients' daily lives in the absence of proper treatment and care. Therefore, we are excited to achieve the first EC approval for an omalizumab biosimilar in Europe, marking a significant step toward enhancing patient access to treatment. As we broaden our biosimilar portfolio globally, specifically in immunology and oncology, we are committed to making a substantial impact on patients battling immunological conditions," stated Hyoung-Ki Kim, Vice Chairman at Celltrion.
Omlyclo® is Celltrion’s sixth biosimilar, alongside Remsima® SC, a subcutaneous form of infliximab, already approved in the EU. This follows previous approvals of Remsima® (infliximab biosimilar), Truxima® (rituximab biosimilar), Herzuma® (trastuzumab biosimilar), Yuflyma® (adalimumab biosimilar), and Vegzelma® (bevacizumab biosimilar). Currently, Omlyclo® is under review by the U.S. Food and Drug Administration, following its submission in March 2024.
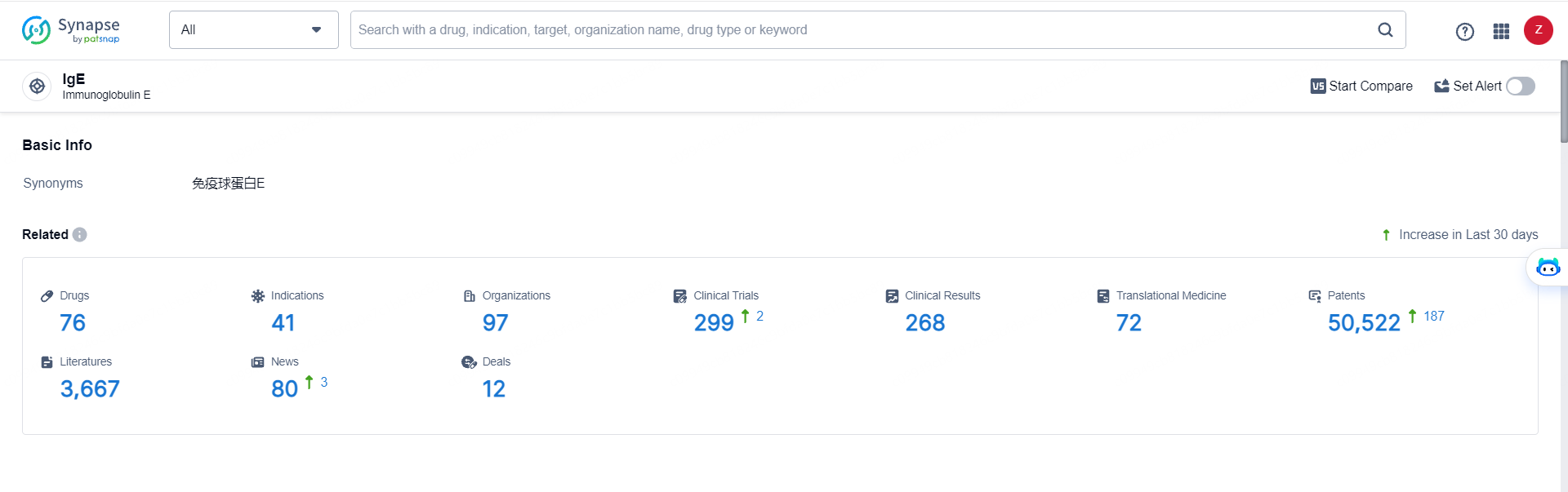
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of May 28, 2024, there are 76 investigational drugs for the IgE target, including 41 indications, 97 R&D institutions involved, with related clinical trials reaching 299, and as many as 50522 patents.
Omlyclo® is the first European Commission approved anti-IgE antibody biosimilar referencing Xolair® (omalizumab). In the EU, Omlyclo® is indicated for the treatment of patients with allergic asthma, chronic spontaneous urticaria and chronic rhinosinusitis with nasal polyps.