Aurinia Reports Initial Participant Dosed in AUR200 Single-Dose Trial
Aurinia Pharmaceuticals Inc. (NASDAQ: AUPH), known as Aurinia or the Company, recently disclosed that the first participant has received the initial dose in a Phase 1a single ascending dose (SAD) clinical trial for AUR200. This experimental treatment aims to be a potentially more effective option for autoimmune disorders, by targeting both BAFF (B-cell Activating Factor) and APRIL (A Proliferation-Inducing Ligand).
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The SAD investigation seeks to assess AUR200's safety, tolerability, pharmacokinetics, and biomarker changes in healthy individuals, with findings expected in the first half of 2025.
"The initiation of the single ascending dose trial is a pivotal moment for AUR200's development, potentially positioning it as a groundbreaking treatment for autoimmune diseases with high unmet needs. Our preclinical studies indicate that AUR200 has superior binding affinity and effectiveness compared to the other TACI-Fc molecules targeting both BAFF and APRIL. We anticipate that this initial trial will provide essential differentiation metrics to inform further clinical progress," commented Dr. Greg Keenan, Chief Medical Officer at Aurinia.
The Company intends to develop AUR200 for conditions with limited current therapies, including a major indication and a smaller, faster-to-market indication that meets FDA criteria for orphan and rare diseases. Detailed indications and development strategies will be announced as early-phase clinical trials progress and as the competitive landscape in relevant therapeutic areas is evaluated. The Company plans to fund this development from current cash flow without altering the previously stated post-restructuring operating expense targets.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
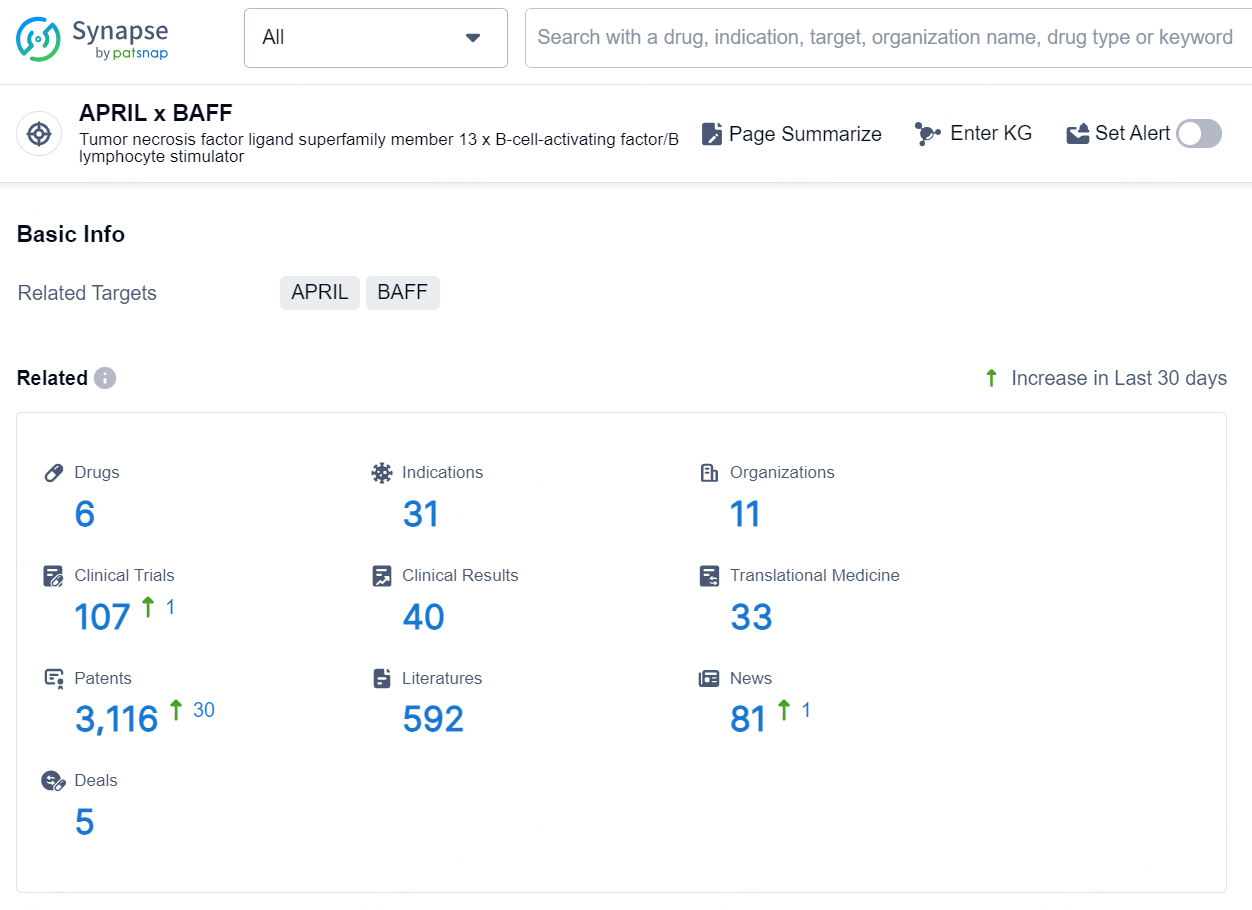
According to the data provided by the Synapse Database, As of September 9, 2024, there are 6 investigational drugs for the APRIL x BAFF targets, including 31 indications, 11 R&D institutions involved, with related clinical trials reaching 107, and as many as 3116 patents.
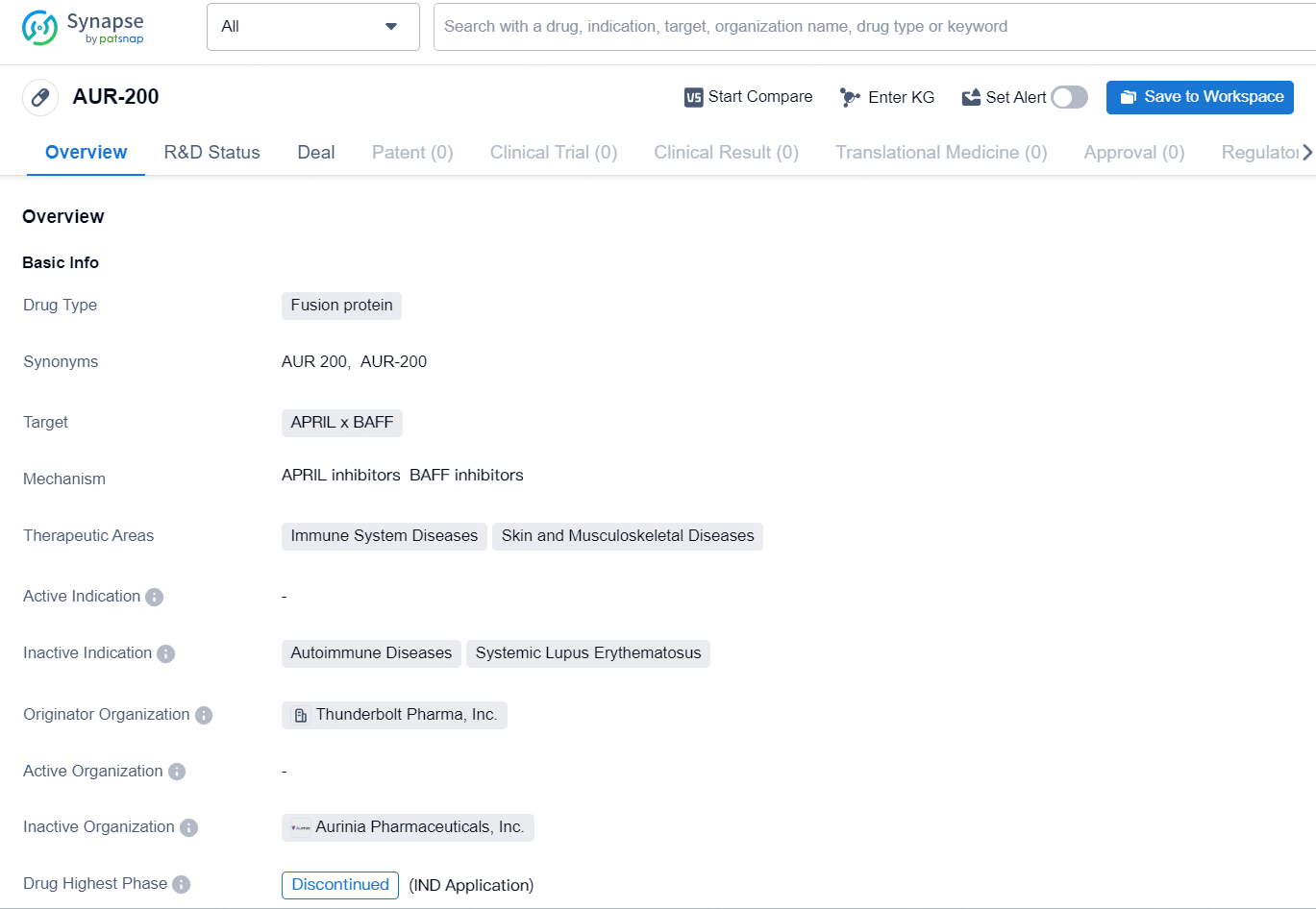
AUR-200 is a fusion protein drug that targets APRIL x BAFF and is indicated for the treatment of immune system diseases, skin, and musculoskeletal diseases. The drug was developed by Thunderbolt Pharma, Inc., an organization specializing in the pharmaceutical industry. However, despite reaching the highest phase of development on a global scale, AUR-200 has been discontinued.