Avirmax Biopharma Doses First Patient in ABI-110 Gene Therapy Study for Wet AMD with PCV
Avirmax Biopharma, Inc., a frontrunner in pioneering gene therapy development, revealed that the initial patient has been successfully treated in the Phase I/IIa clinical study of ABI-110, the company’s inaugural gene therapy aimed at addressing Wet Age-related Macular Degeneration (AMD) along with Polypoidal Choroidal Vasculopathy (PCV).
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
"We are excited to share this important achievement in the clinical study of ABI-110," stated Shawn Liu, Ph.D., Chief Executive Officer of Avirmax Biopharma Inc. "ABI-110 holds the promise to transform the therapeutic options for Wet AMD and PCV."
Avirmax has administered the initial dose to its first patient in the Phase I/IIa trial of ABI-110, a groundbreaking gene therapy for Wet AMD and PCV.
"Initiating dosing for the first patient represents a significant advancement for Avirmax’s goal of delivering innovative gene therapies to individuals affected by serious retinal disorders," remarked Wendy Murahashi, M.D., Chief Medical Officer of Avirmax Biopharma Inc.
Wet Age-related Macular Degeneration (AMD) and Polypoidal Choroidal Vasculopathy (PCV) are severe retinal disorders that may result in substantial vision impairment. Existing treatment options often necessitate regular injections and offer only short-term relief. ABI-110 could potentially provide a more effective and lasting solution by targeting the underlying genetic factors responsible for these conditions.
ABI-110, the proprietary gene therapy developed by Avirmax Biopharma, employs an engineered capsid, AAV2.N54, for the efficient delivery of the therapeutic transgene to the macular retina. This strategy aims to create a long-lasting remedy that surpasses the current treatment capabilities. The Phase 1 clinical trial is intended to assess the safety, tolerability, and initial effectiveness of ABI-110 in patients diagnosed with Wet AMD and PCV.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of November 27, 2024, there are 243 investigational drugs for the Wet age-related macular degeneration, including 86 targets, 261 R&D institutions involved, with related clinical trials reaching 810, and as many as 5480 patents.
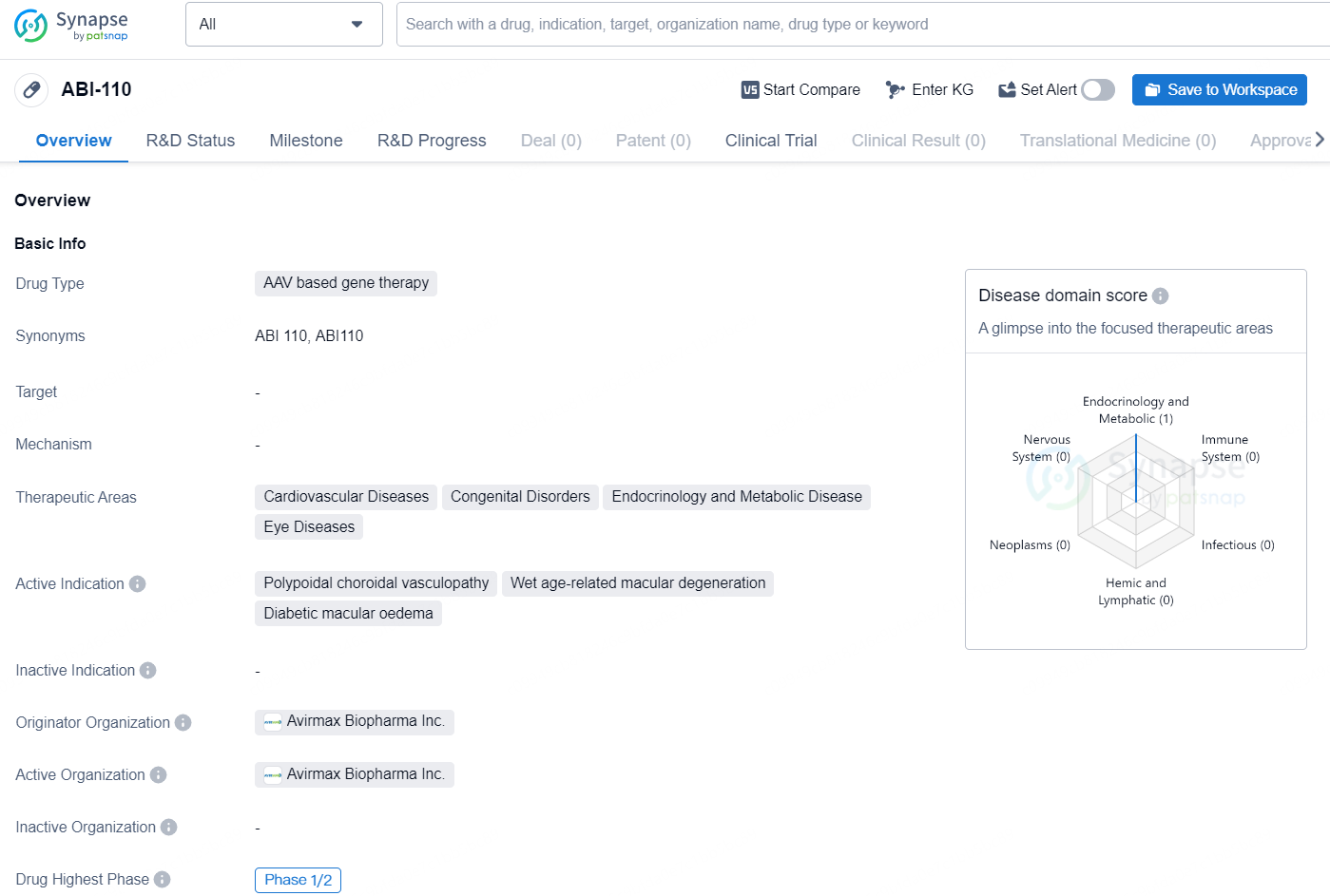
ABI-110 is a gene therapy drug developed by Avirmax Biopharma Inc., targeting therapeutic areas such as cardiovascular diseases, congenital disorders, endocrinology and metabolic disease, as well as eye diseases. The active indications for ABI-110 include polypoidal choroidal vasculopathy, wet age-related macular degeneration, and diabetic macular edema. The drug falls under the category of AAV-based gene therapy.