Axalbion Therapeutics Initiates Phase 2 Trial of New TRPM8 Agonist AX-8 in Chronic Cough Patients
Axalbion, a biopharmaceutical company at the clinical stage concentrating on innovative treatments for cough, disclosed that the second segment of a Phase 2 proof-of-concept randomized, placebo-controlled clinical trial in chronic cough has commenced with the dosing of the first patient. This study involves AX-8, their leading compound, which is a powerful and selective oral agonist of the transient receptor potential melastatin 8 (TRPM8) ion channel. To aid in the execution of this trial and the preparation for Phase 3, the company has secured further funding from both current and new investors.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Michael Kitt, MD, chief executive officer of Axalbion, declared, “We are excited to reveal that Axalbion has commenced the second segment of its Phase 2 clinical trial with AX-8 for treating chronic cough, a condition with substantial unmet needs.” “In the initial segment of this research, AX-8 demonstrated encouraging outcomes by significantly and consistently reducing cough frequency, especially in patients who experienced severe throat discomfort—a common and troubling symptom in chronic cough sufferers.”
The findings were shared in an oral presentation at the American Thoracic Society (ATS) 2023 International Conference.
Based on the early positive results, the company has progressed to Part 2 of the AX-8 study in chronic cough patients who have moderate-to-severe throat discomfort (defined as having a throat discomfort visual analog scale score ≥50mm out of 100mm), as these patients are likely to benefit the most from AX-8 treatment. The Phase 2 trial, focusing on about 50 patients with refractory or unexplained chronic cough (RCC/UCC), follows a randomized, double-blind, placebo-controlled, crossover design to evaluate both the efficacy and safety of AX-8.
Patients will be administered 40 mg of AX-8 or a matching placebo three times daily, spaced roughly four hours apart (compared to twice daily in Part 1), over the course of two weeks. This will be followed by a seven-day washout period before they switch to the other treatment for another two weeks. AX-8 is provided as an orally disintegrating tablet placed on the tongue. Cough frequency will be measured using an objective cough recording device. The primary endpoint is the placebo-adjusted change in cough frequency from baseline. The study will be conducted across multiple sites in the U.K. and is anticipated to conclude by the latter half of 2025. Additional details can be found at clinicaltrials.gov: NCT04866563.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
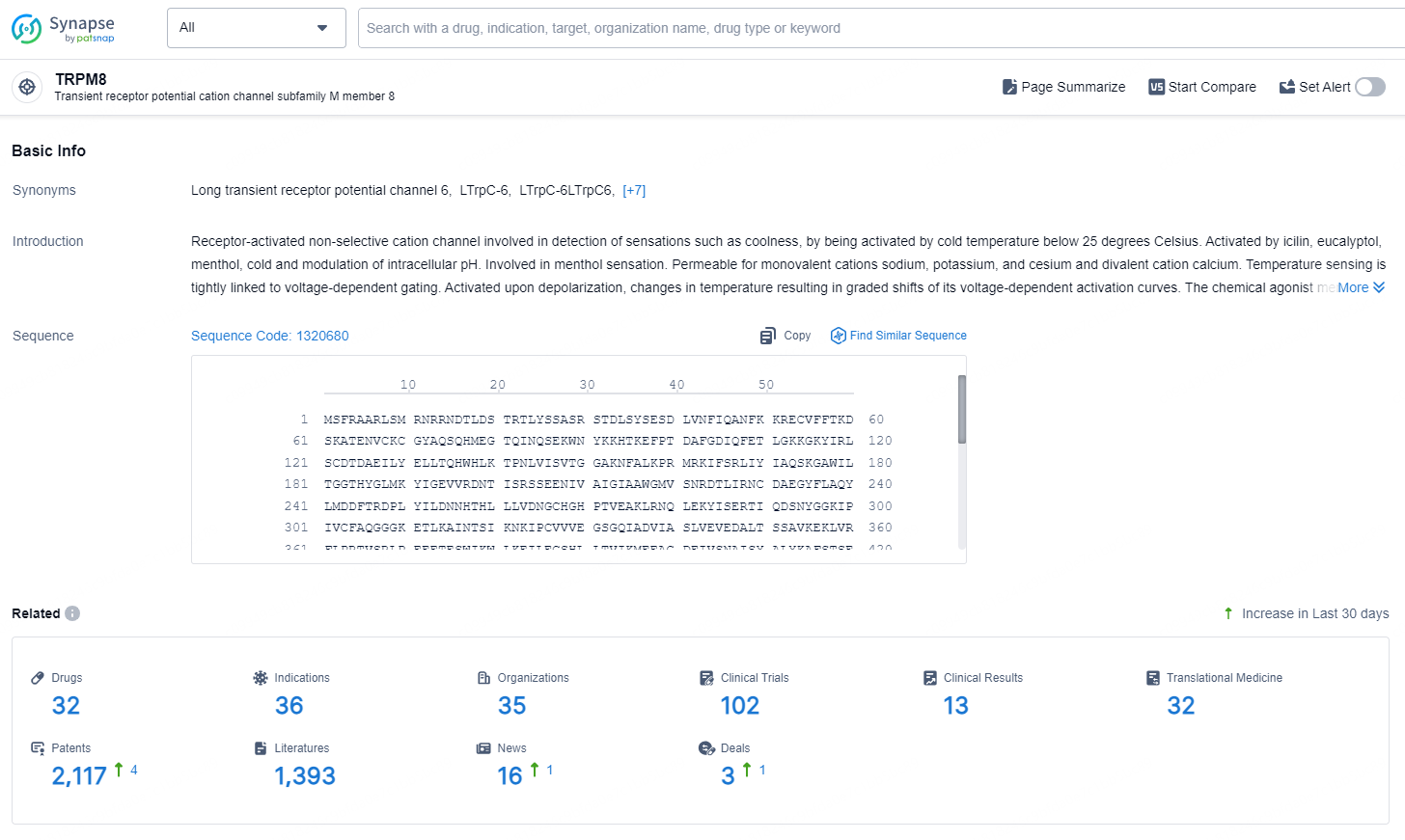
According to the data provided by the Synapse Database, As of August 29, 2024, there are 32 investigational drugs for the TRPM8 targets, including 36 indications, 35 R&D institutions involved, with related clinical trials reaching 102, and as many as 2117 patents.
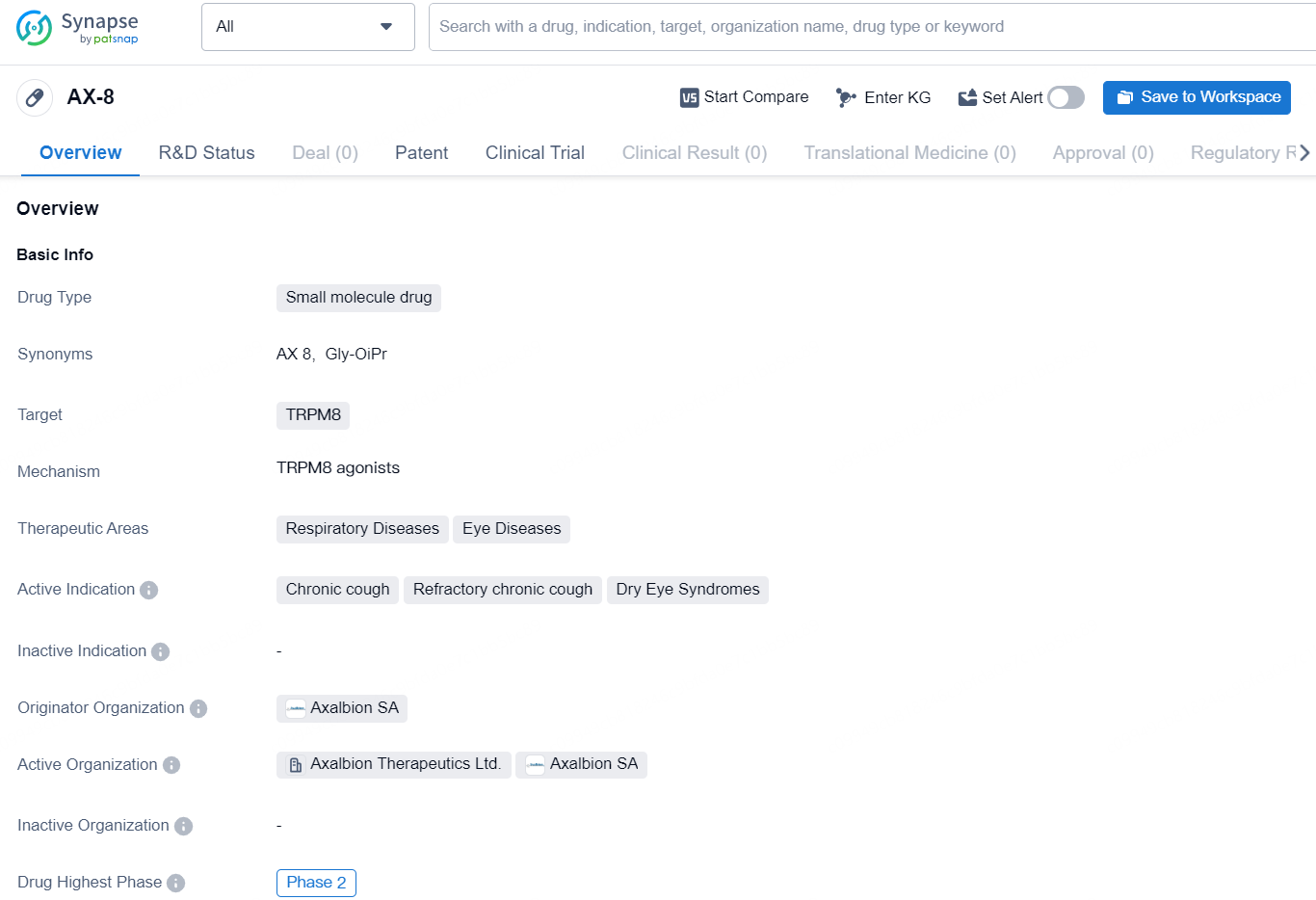
The pharmaceutical industry expert specializes in Business Development reports within the field of biomedicine. In this context, the drug AX-8 is a small molecule drug that targets TRPM8. Its therapeutic areas include respiratory diseases and eye diseases, and it is actively indicated for chronic cough, refractory chronic cough, and dry eye syndromes. The drug originates from Axalbion SA and has reached the highest global phase of Phase 2 in its development.