Bcl-2 inhibitors show promise for the treatment of various hematological malignancies
The Bcl-2 protein family plays a critical role in the endogenous cell apoptosis pathway. The members of the Bcl-2 protein family can be divided into three functional groups: anti-apoptotic proteins (such as Bcl-2 and Bcl-xL), pro-apoptotic effectors, and pro-apoptotic activators. When the effectors and activators come into direct contact with anti-apoptotic Bcl-2 family members, their apoptotic promoting effects will be inhibited. In preclinical models, it has been found that Bcl-2 protein family can directly inhibit effectors, or bind with activators and isolate them to prevent their interaction with effectors.
The dynamic balance between anti-apoptotic members (such as Bcl-2) and pro-apoptotic members determines whether the cell begins apoptosis. In different types of tumors, Bcl-2 is found to be overexpressed, with Bcl-2 protein preventing tumor cell apoptosis. In terms of solid tumors, overexpression of Bcl-2 has been observed in prostate cancer, breast cancer, and both small cell and non-small cell lung cancer, indicating a possible link between Bcl-2 and these tumors. Bcl-2 overexpression may also be linked to hematological cancers, and several inhibitors of the Bcl-2 family have been clinically developed for certain malignant hematological tumors, such as leukemia and lymphoma.
The Bcl-2 gene is often overexpressed in cells that divide or differentiate rapidly. As cells enter programmed death, this gene expression decreases. If the Bcl-2 gene is highly expressed during cell apoptosis, it will reduce the release of cytochrome C and SMAC in the apoptosis process, inhibiting cell programmed death and causing uncontrollable cell proliferation, leading to the development of cancer. Bcl-2 inhibitors can restore normal cell apoptosis process by inhibiting the formation of Bcl-2 and Bax protein dimers. Bcl-2 inhibitors can be used alone to kill cancer cells or can be used in combination with other drugs to increase the sensitivity of cancer cells to drugs.
Bcl-2 Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 8 Sep 2023, there are a total of 64 Bcl-2 drugs worldwide, from 93 organizations, covering 96 indications, and conducting 826 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.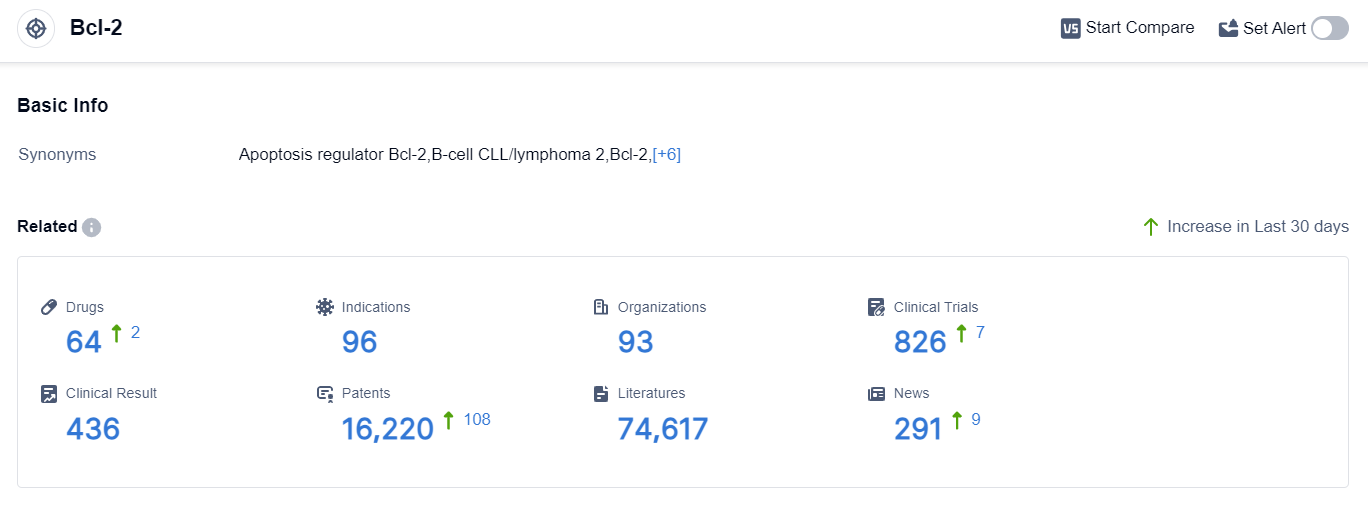
The analysis of the target Bcl-2 reveals a competitive landscape with multiple companies actively involved in the research and development of drugs. AbbVie, Inc., Ascentage Pharma Group International Co., Ltd., and Roche Holding AG are among the companies growing fastest under this target.
Drugs targeting Bcl-2 have been approved for various indications, primarily in the field of oncology. Small molecule drugs are progressing most rapidly, indicating intense competition around innovative drug development.
China is emerging as a key player in the development of drugs targeting Bcl-2, with significant progress in R&D. Overall, the target Bcl-2 presents a promising opportunity for the pharmaceutical industry, particularly in the field of cancer therapeutics.
The only globally approved Bcl-2 inhibitor on the market: Venetoclax
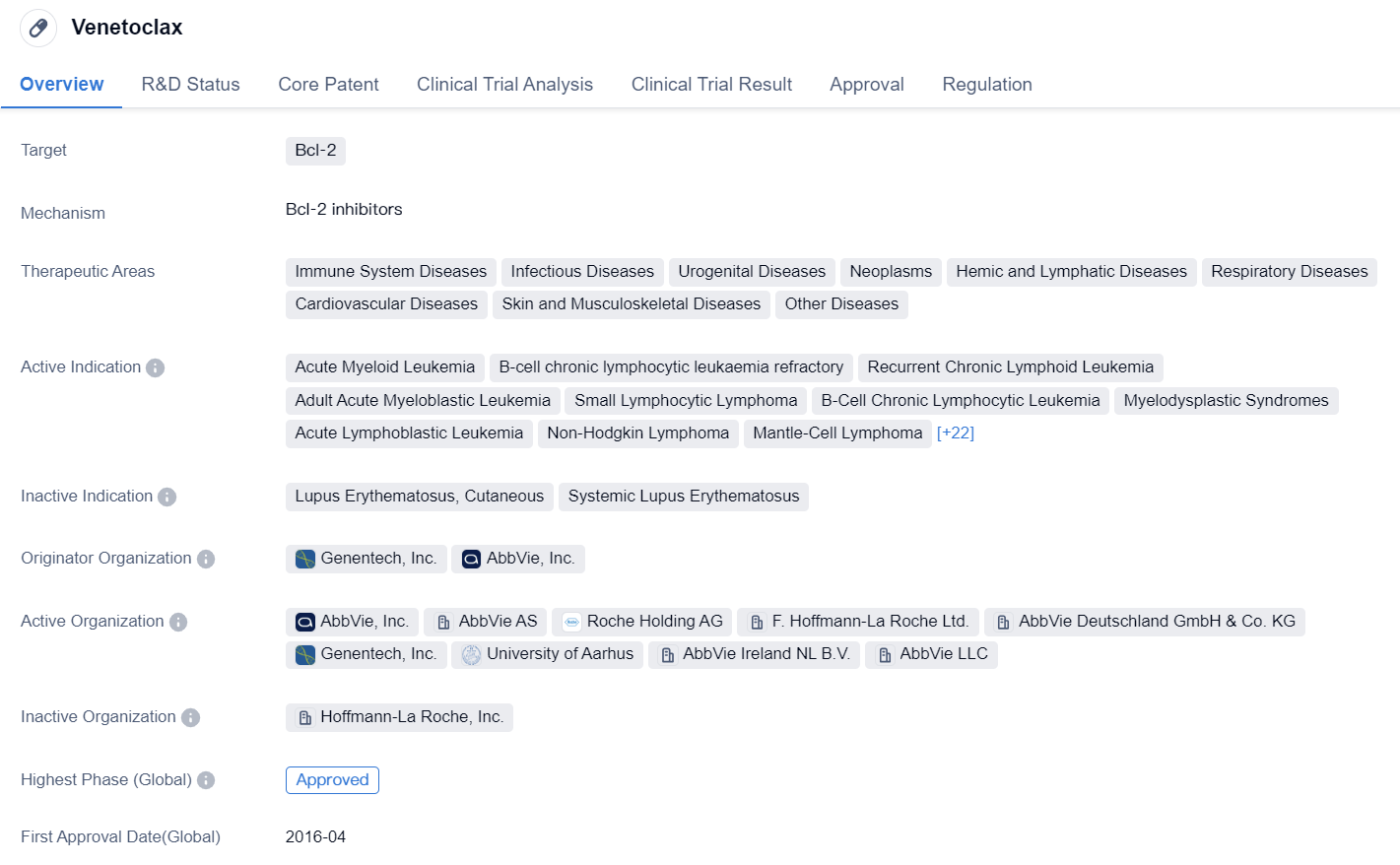
Venetoclax is a small molecule drug that targets Bcl-2, a protein involved in regulating cell death. It has been approved for use in various therapeutic areas, including immune system diseases, infectious diseases, urogenital diseases, neoplasms, hemic and lymphatic diseases, respiratory diseases, cardiovascular diseases, skin and musculoskeletal diseases, and other diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The drug has shown efficacy in treating several indications, including acute myeloid leukemia, B-cell chronic lymphocytic leukemia refractory, recurrent chronic lymphoid leukemia, adult acute myeloblastic leukemia, small lymphocytic lymphoma, myelodysplastic syndromes, acute lymphoblastic leukemia, non-Hodgkin lymphoma, mantle-cell lymphoma, multiple myeloma, chronic myelogenous leukemia, marginal zone B-cell lymphoma, neuroblastoma, solid tumors, T-cell prolymphocytic leukemia, breast cancer, follicular lymphoma, diffuse large B-cell lymphoma, Waldenstrom macroglobulinemia, bone marrow neoplasms, chromosome deletion, chronic myelomonocytic leukemia, prostatic cancer, kidney diseases, blastic plasmacytoid dendritic cell neoplasm, B-cell lymphoma, hematologic neoplasms, non-small cell lung cancer, small cell lung cancer, and HIV-1 infection.
Venetoclax was developed by Genentech, Inc. and AbbVie, Inc. It received its first approval in the United States in April 2016. The drug has also been approved in China. It has gone through various regulatory pathways, including accelerated approval, orphan drug designation, breakthrough therapy designation, and special review project.
In summary, Venetoclax is a small molecule drug that targets Bcl-2 and has been approved for multiple indications in various therapeutic areas. It has shown promise in treating a wide range of diseases, including different types of leukemia, lymphoma, solid tumors, and HIV-1 infection. Developed by Genentech, Inc. and AbbVie, Inc., the drug received its first approval in the United States in 2016 and has also been approved in China. Its development has been supported by regulatory pathways such as accelerated approval, orphan drug designation, breakthrough therapy designation, and special review project.
Bcl-2 inhibitor at clinical Phase II: Lisaftoclax
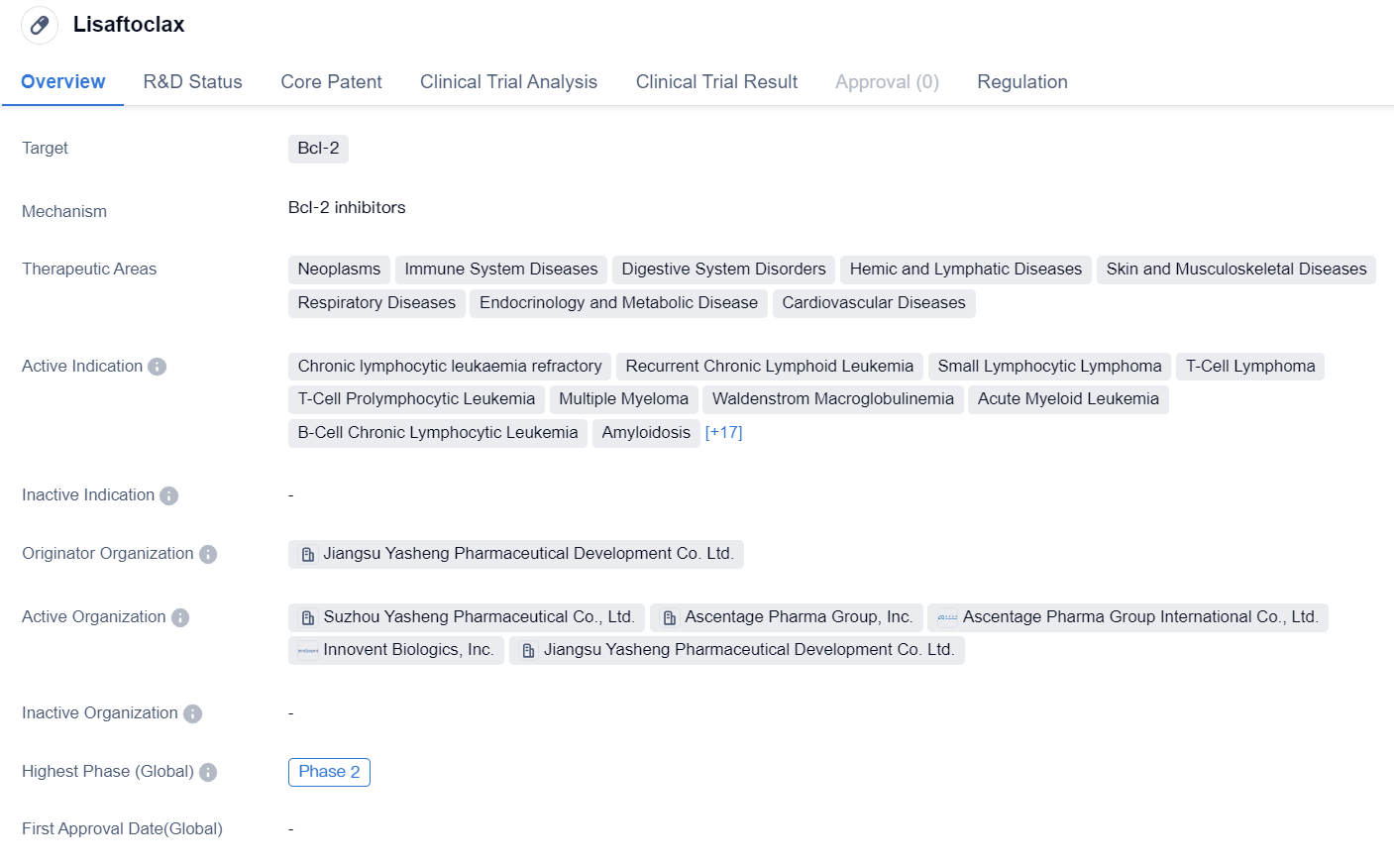
Lisaftoclax is a small molecule drug that targets Bcl-2, a protein involved in regulating cell death. It has shown potential therapeutic benefits in various therapeutic areas including neoplasms, immune system diseases, digestive system disorders, hemic and lymphatic diseases, skin and musculoskeletal diseases, respiratory diseases, endocrinology and metabolic diseases, and cardiovascular diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The drug is developed by Jiangsu Yasheng Pharmaceutical Development Co. Ltd., an originator organization in the pharmaceutical industry. Currently, Lisaftoclax has reached Phase 2 of clinical development globally and in China.
Lisaftoclax has been designated as an orphan drug, indicating its potential to treat rare diseases or conditions. This regulatory status provides certain incentives and benefits to the drug's development and commercialization.
In summary, Lisaftoclax is a small molecule drug that targets Bcl-2 and has shown potential therapeutic benefits in various therapeutic areas. It is indicated for the treatment of multiple conditions, including various types of leukemia, lymphoma, solid tumors, and other diseases. Developed by Jiangsu Yasheng Pharmaceutical Development Co. Ltd., the drug has reached Phase 2 of clinical development globally and in China. Its orphan drug designation highlights its potential to address unmet medical needs in rare diseases or conditions.