Beacon Therapeutics Releases 36-Month Data on AGTC-501 from HORIZON Phase I/2 Trial for XLRP
Beacon Therapeutics Holdings Limited (‘Beacon’), a prominent ophthalmic gene therapy firm dedicated to preserving and restoring vision in individuals suffering from blinding retinal diseases, unveiled 36-month interim findings from its Phase I/2 HORIZON trial of AGTC-501, its flagship product, targeting patients with X-linked retinitis pigmentosa (XLRP) at the 24th EURETINA Congress in Barcelona, Spain.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Key presentation highlights included:
- AGTC-501 was found to be generally safe and well-tolerated among the 29 patients enrolled, with no clinically significant safety concerns related to the therapy.
- Evidence showed that a difference in visual function between the treated and untreated eyes persisted at the 36-month mark.
- The benefit-risk profile of AGTC-501 supports its continued clinical development for treating patients with XLRP caused by RPGR mutations.
Lance Baldo, MD, Chief Executive Officer of Beacon, commented, “This emerging longer-term data further validates the safety of AGTC-501 for treating XLRP. We are excited about the upcoming clinical milestones, including the 24-month data from the Phase 2 SKYLINE trial in XLRP expected later this year, and the ongoing enrollment in our open-label Phase 2 DAWN trial and Phase 2/3 VISTA trial.”
HORIZON is a Phase 1/2, open-label, dose-escalation study involving XLRP patients treated with subretinal AGTC-501. This study has completed the enrollment of 29 male participants, all of whom are now in long-term follow-up.
Presentation – Subretinal Gene Therapy Drug AGTC-501 for X-Linked Retinitis Pigmentosa (XLRP) Phase 1/2 Multicenter Study (HORIZON): 36-Month Interim Results
Presenter – Paul Yang, MD, PhD, Chief, Paul H. Casey Ophthalmic Genetics Division, Casey Eye Institute, OHSU
The presentation was held on Thursday, September 19th at 15:30 CEST.
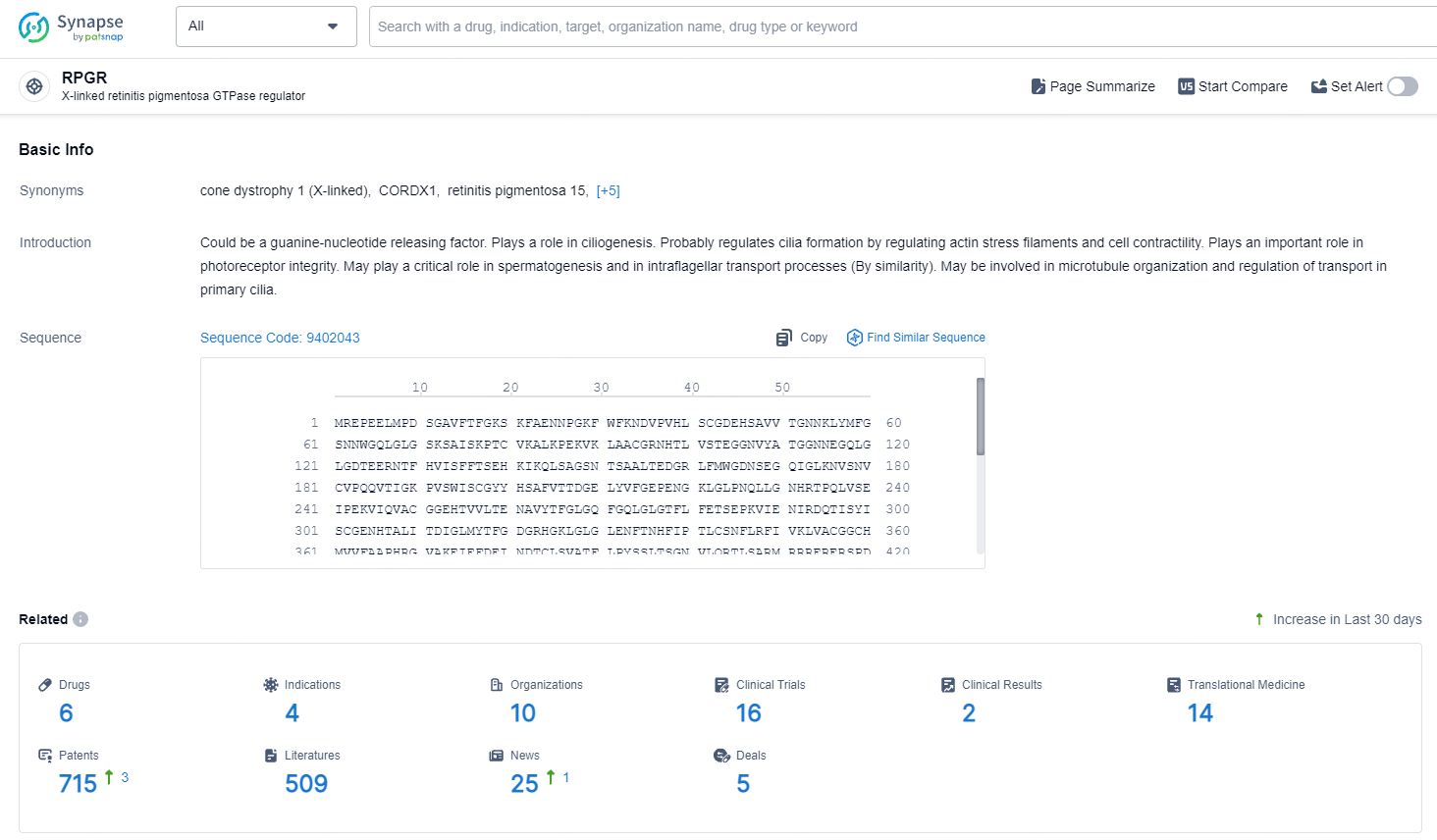
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 23, 2024, there are 6 investigational drugs for the RPGR targets, including 4 indications, 10 R&D institutions involved, with related clinical trials reaching 16, and as many as 715 patents.
Laruparetigene zosaparvovec is an AAV-based gene therapy drug developed by Beacon Therapeutics (USA), Inc. The drug is designed to target RPGR, a gene associated with X-linked retinitis pigmentosa, a rare genetic disorder that causes progressive vision loss. The therapeutic areas for this drug include congenital disorders, eye diseases, and other diseases. Laruparetigene zosaparvovec has reached the highest phase of development globally, which is Phase 2/3. In terms of regulation, it has been granted priority review and fast track status, indicating the potential for expedited approval and market access.