RYBREVANT® Approved in U.S. as Exclusive Second-Line Targeted Treatment for EGFR-Mutated Advanced Lung Cancer
Johnson & Johnson (NYSE: JNJ) revealed that the U.S. Food and Drug Administration (FDA) has given approval for RYBREVANT® (amivantamab-vmjw) in combination with the standard chemotherapy regimen (carboplatin and pemetrexed) for the management of adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) displaying epidermal growth factor receptor (EGFR) exon 19 deletions (ex19del) or L858R substitution mutations, whose illness has progressed following treatment with an EGFR tyrosine kinase inhibitor (TKI).
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"RYBREVANT plus chemotherapy may potentially overcome the primary mechanisms of treatment resistance to third-generation EGFR TKIs, including osimertinib, when used as a first-line treatment," stated Martin Dietrich, M.D., Ph.D., an Oncologist at Cancer Care Centers of Brevard. "This combination therapy has been shown to extend progression-free survival and boost overall response rates compared to chemotherapy alone, presenting a valuable and effective second-line treatment option for patients."
The five-year survival rate for individuals with advanced EGFR-mutated NSCLC is under 20 percent. The mechanisms of acquired resistance following TKI monotherapy are varied and polyclonal, complicating targeted treatments upon progression and thereby limiting their effectiveness. Additionally, combining immunotherapy with chemotherapy has not yielded significant clinical improvements.
"The progression-free survival improvements observed in the MARIPOSA-2 study are very encouraging," commented Andrea Ferris, President and CEO of the LUNGevity Foundation. "It's promising to see treatment combinations like RYBREVANT and chemotherapy addressing unmet needs in EGFR-mutated lung cancer and offering hope for positive outcomes to more patients and their loved ones."
FDA approval was granted based on the outcomes from the Phase 3 MARIPOSA-2 (NCT04988295) trial, which assessed the efficacy and safety of RYBREVANT® combined with chemotherapy for adult patients with locally advanced or metastatic NSCLC harboring EGFR ex19del or L858R mutations after disease progression on or post-osimertinib treatment. Findings indicated that RYBREVANT® plus chemotherapy reduced the risk of disease progression or death (progression-free survival [PFS]) by 52 percent compared to chemotherapy alone, with the median PFS for the combination being 6.3 months versus 4.2 months for chemotherapy only. Additionally, the overall response rate (ORR) for the combination was 53 percent, compared to 29 percent with chemotherapy alone.
Amivantamab-vmjw (RYBREVANT®) in combination with chemotherapy is the sole National Comprehensive Cancer Network® (NCCN®) Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Category 1 recommended treatment for patients with EGFR-mutated NSCLC progressing on osimertinib who present symptoms with multiple lesions.
"This achievement underscores the significance of RYBREVANT as a treatment option for patients with EGFR-mutated NSCLC facing high unmet needs following progression on TKI therapy," said Kiran Patel, M.D., Vice President, Clinical Development, Solid Tumors, Johnson & Johnson Innovative Medicine. "Patients deserve and require effective, targeted treatments across all stages of therapy. Through RYBREVANT-based regimens, we are introducing potential new standards of care for the approximately 30,000 patients diagnosed with EGFR-mutated NSCLC in the United States each year."
The safety profile of the RYBREVANT® and chemotherapy combination aligned with the known profiles of each individual treatment. Permanent discontinuation of RYBREVANT® due to adverse reactions was observed in 11 percent of patients.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
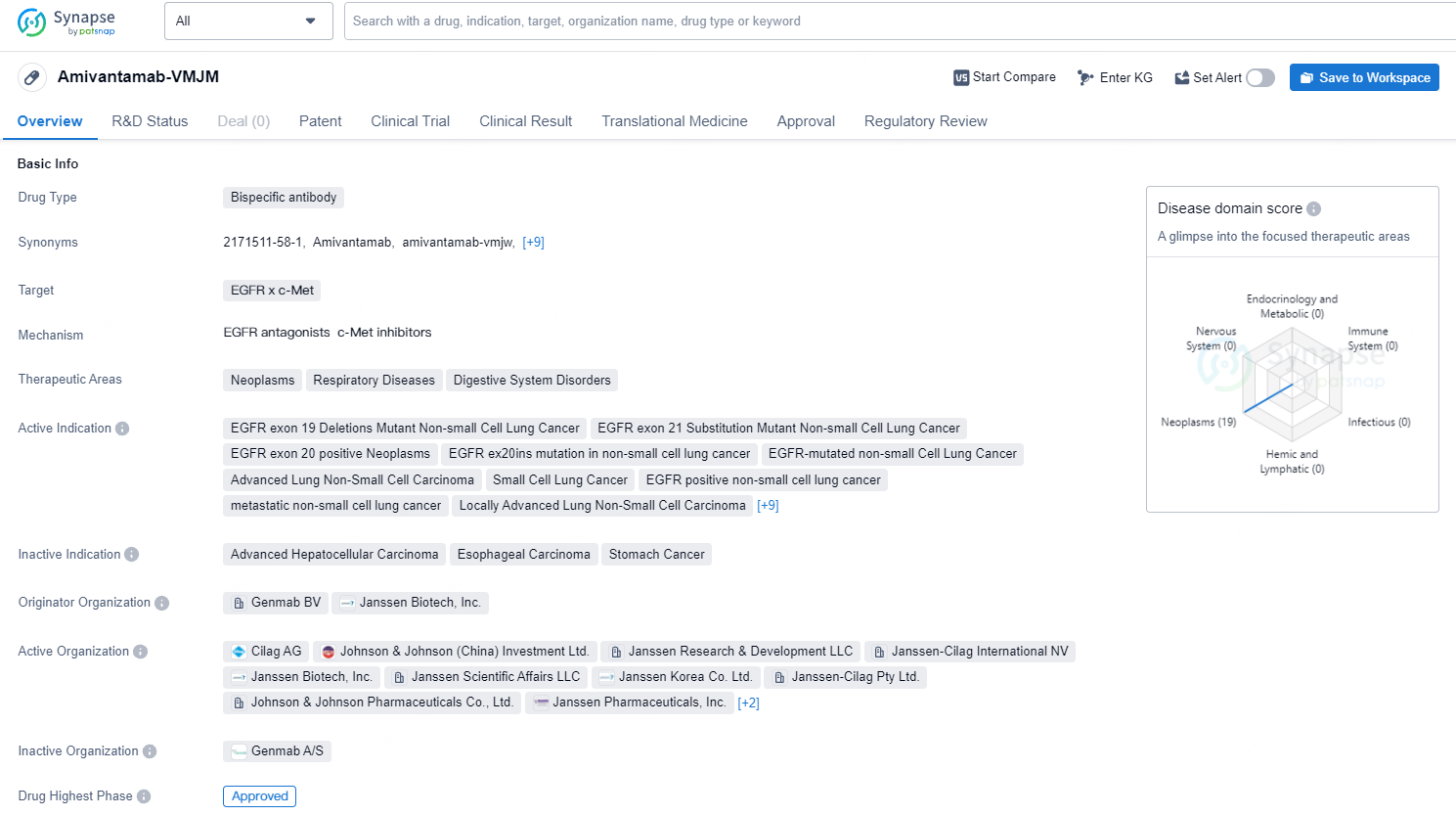
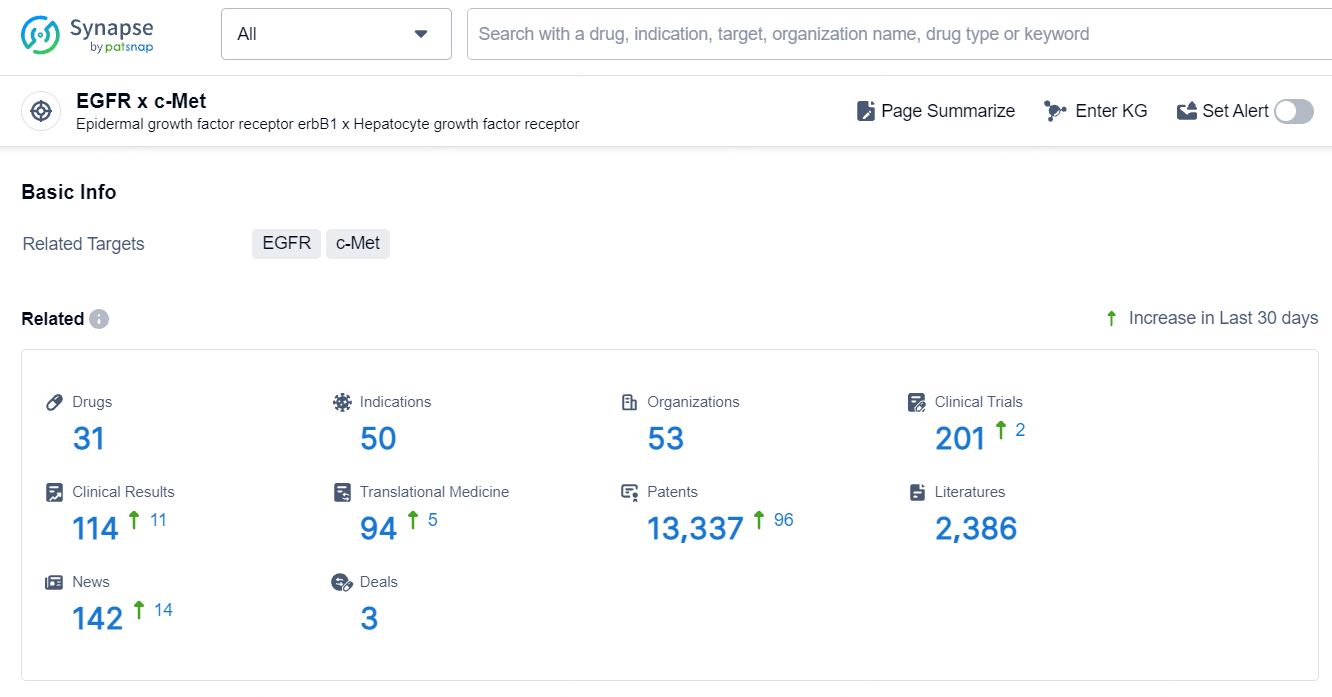
According to the data provided by the Synapse Database, As of September 23, 2024, there are 31 investigational drug for the EGFR and c-Met targets, including 50 indications, 53 R&D institutions involved, with related clinical trials reaching 201, and as many as 13337 patents.
Amivantamab-VMJM is a bispecific antibody drug that targets the EGFR and c-Met receptors. Its therapeutic areas include neoplasms, respiratory diseases, and digestive system disorders. The drug is indicated for various types of non-small cell lung cancer, including EGFR exon 19 deletions mutant, EGFR exon 21 substitution mutant, EGFR exon 20 positive, and EGFR ex20ins mutation. It is also indicated for advanced lung non-small cell carcinoma, small cell lung cancer, metastatic colorectal carcinoma, and other advanced malignant solid neoplasms. The drug was developed by Genmab BV and Janssen Biotech, Inc. It has received approval in the United States in May 2021 and is currently in the NDA/BLA phase in China. Amivantamab-VMJM has been granted various regulatory designations, including priority review, accelerated approval, breakthrough therapy, and orphan drug status.