Novo Nordisk: A long-acting, selective human insulin analog, NN1213, for weight management
Recently, the Novo Nordisk team published an article in the Journal of Medicinal Chemistry, detailing the development of a long-acting, selective human amylin analog, NN1213, and its preclinical study data for weight management. Amylin is a pancreatic peptide consisting of 37 amino acids that is co-stored and co-secreted with insulin in response to nutrient intake. Post-secretion, amylin activates receptors in the posterior brain and hypothalamus, inducing satiety and affecting glucose control by inhibiting meal-stimulated glucagon secretion (in non-hypoglycemic states) and delaying gastric emptying.
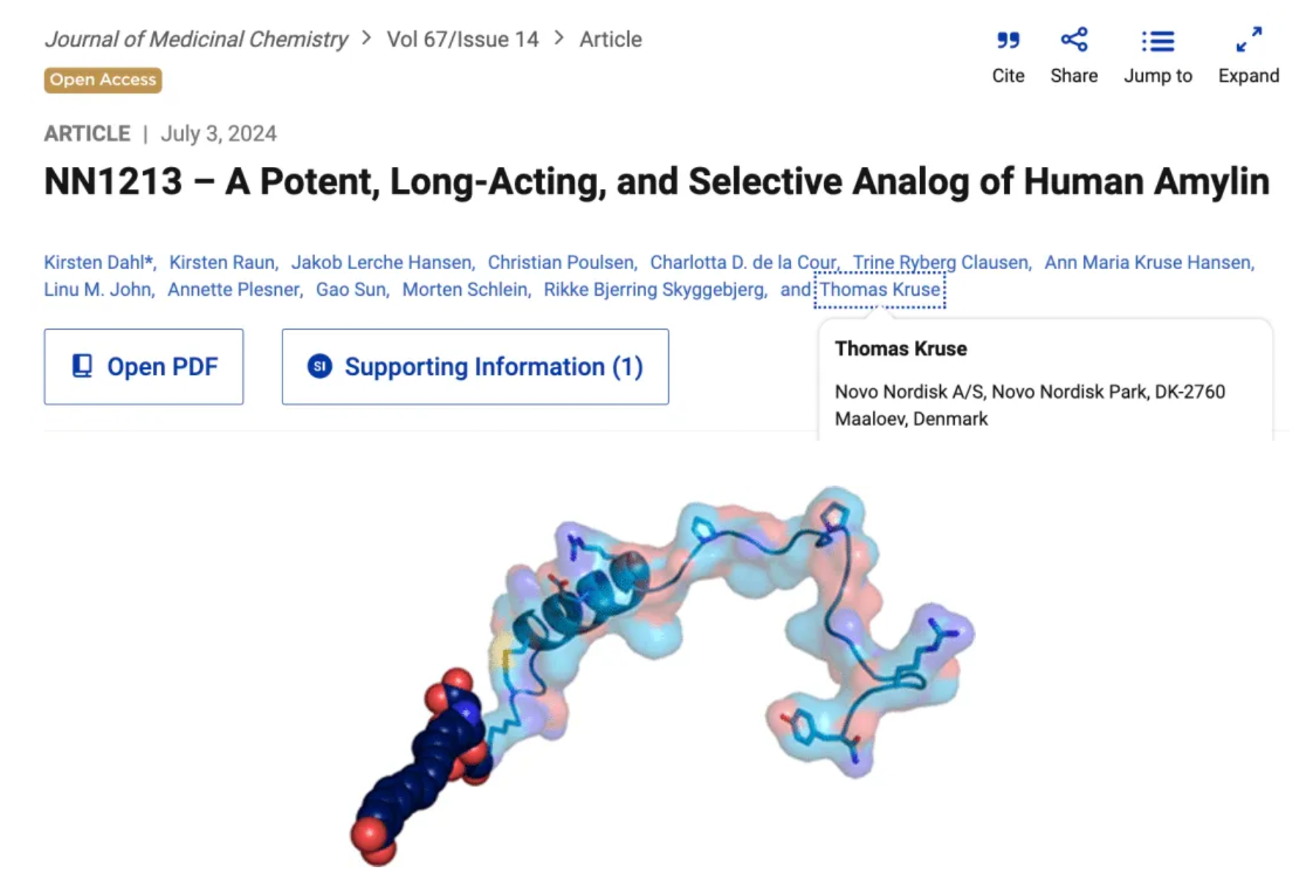
These biological effects support the research and development of optimized amylin analogs for use in weight management and diabetes treatment. Indeed, since 2005, the amylin analog pramlintide (Symlin) has been available on the U.S. market as a supplementation to insulin, helping diabetic patients who need additional blood glucose control support. Moreover, Novo Nordisk's long-acting but non-selective amylin analog, cagrilintide, has been shown to effectively reduce weight, either alone or in combination with a long-acting glucagon-like peptide-1 (GLP-1) analog, semaglutide.
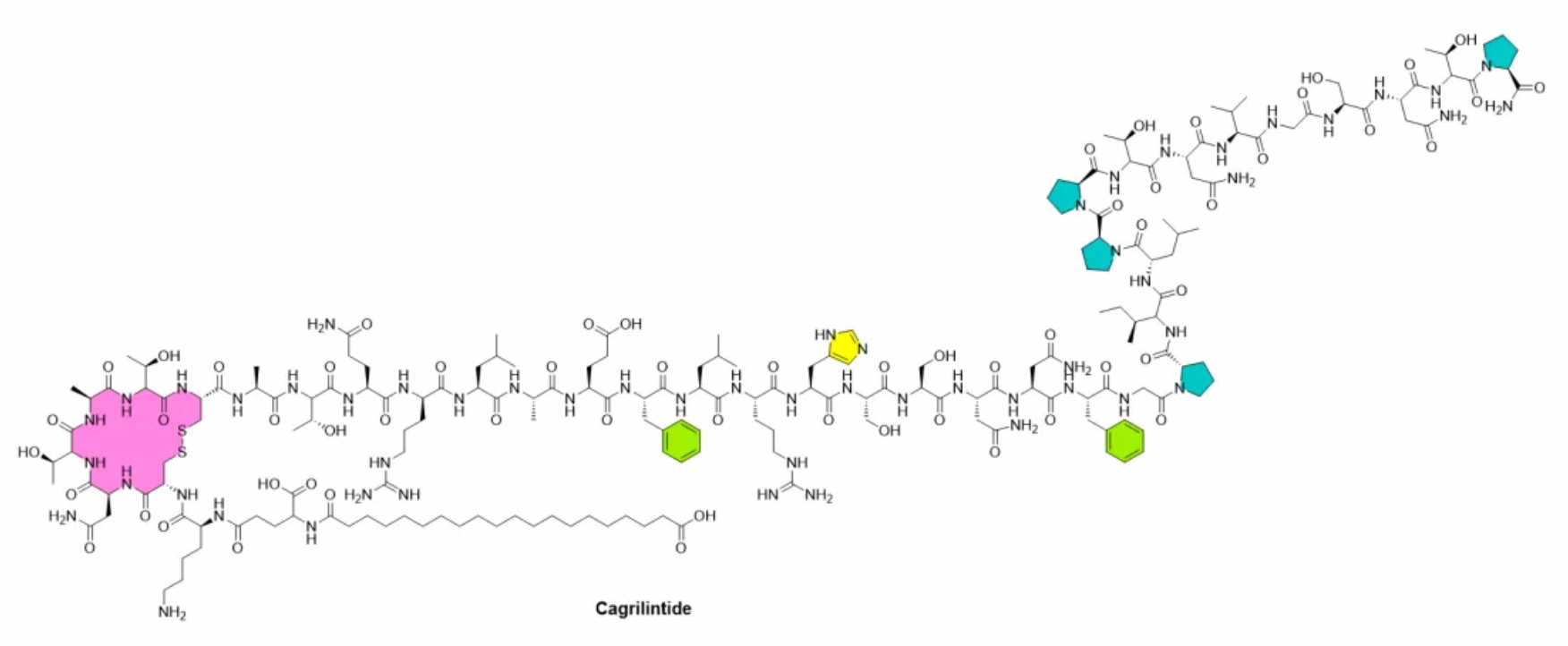
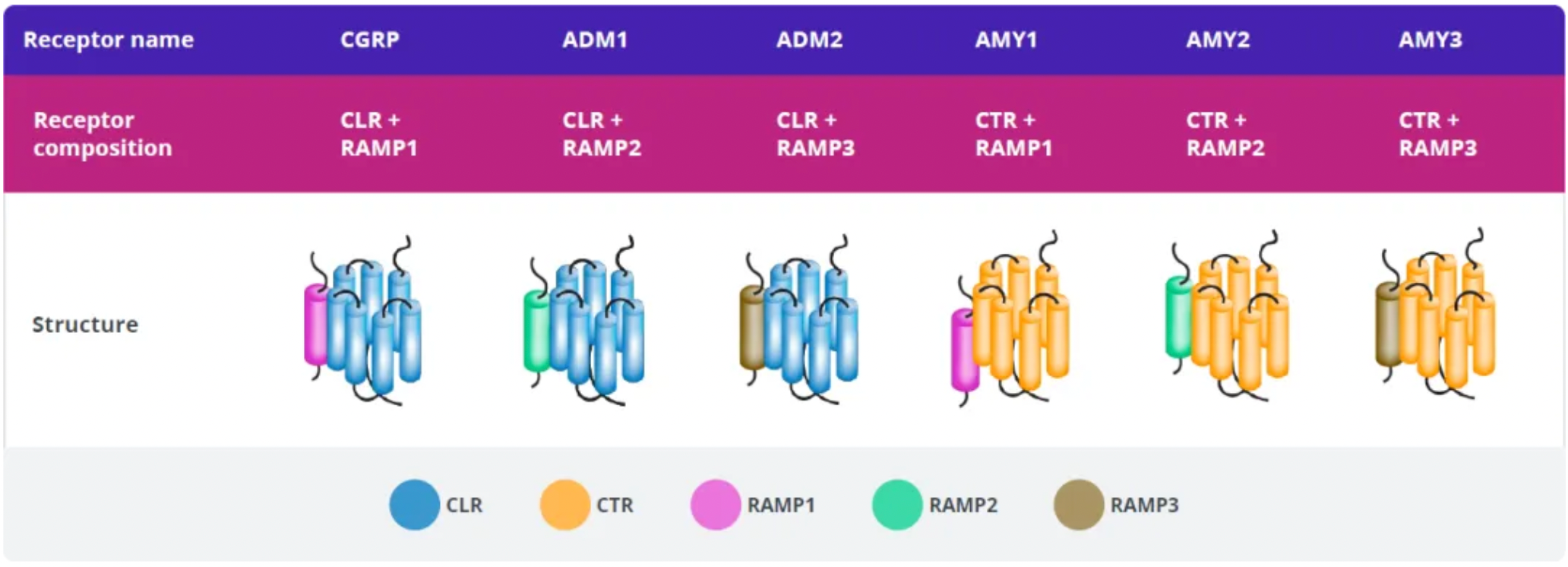
Amylin belongs to the calcitonin peptide family, which also includes calcitonin, α and β-type calcitonin gene-related peptides (CGRP), adrenomedullin 1, and adrenomedullin 2. These peptides act as ligands for a family of G-protein-coupled receptors (GPCRs), including the calcitonin receptor (CTR), a calcitonin receptor-like receptor bound to one of three receptor activity-modifying proteins (RAMP1-3). The peptides and receptors of the calcitonin peptide family are associated with various biological functions including regulating blood flow, bone metabolism, and energy metabolism.
Salmon calcitonin (sCT) shares approximately 50% sequence similarity with mammalian calcitonins and is chemically more stable with a lower propensity for fibril formation. It is a potent and non-selective agonist of AMYR and CTR receptors. sCT is marketed under the names Miacalcin (in the USA) and Miacalcic (in the EU), and has been used for decades to treat postmenopausal osteoporosis, Paget's disease, and other bone disorders.
In addition to achieving AMYR selectivity, a major obstacle in the pharmaceutical development of insulin is the inherent instability of the peptide, including its tendency to form fibrils and chemical degradation, which also needs to be addressed to achieve a molecule with drug-like properties.
The amino acid sequence of human insulin allows the process of misfolding to take place, where monomeric insulin initially forms soluble oligomers rich in β-sheet structures, which might be cytotoxic. Over time, these oligomers may mature into extended structures with a high content of β-sheet chains, eventually forming insoluble protein aggregates that are histologically visible as amyloid fibrils in the islets of Type 2 diabetes patients. These toxic oligomer species are associated with β-cell death and the progression of Type 2 diabetes. Preventing fibril formation and improving β-cell survival and glucose control are related and have been demonstrated in preclinical models.
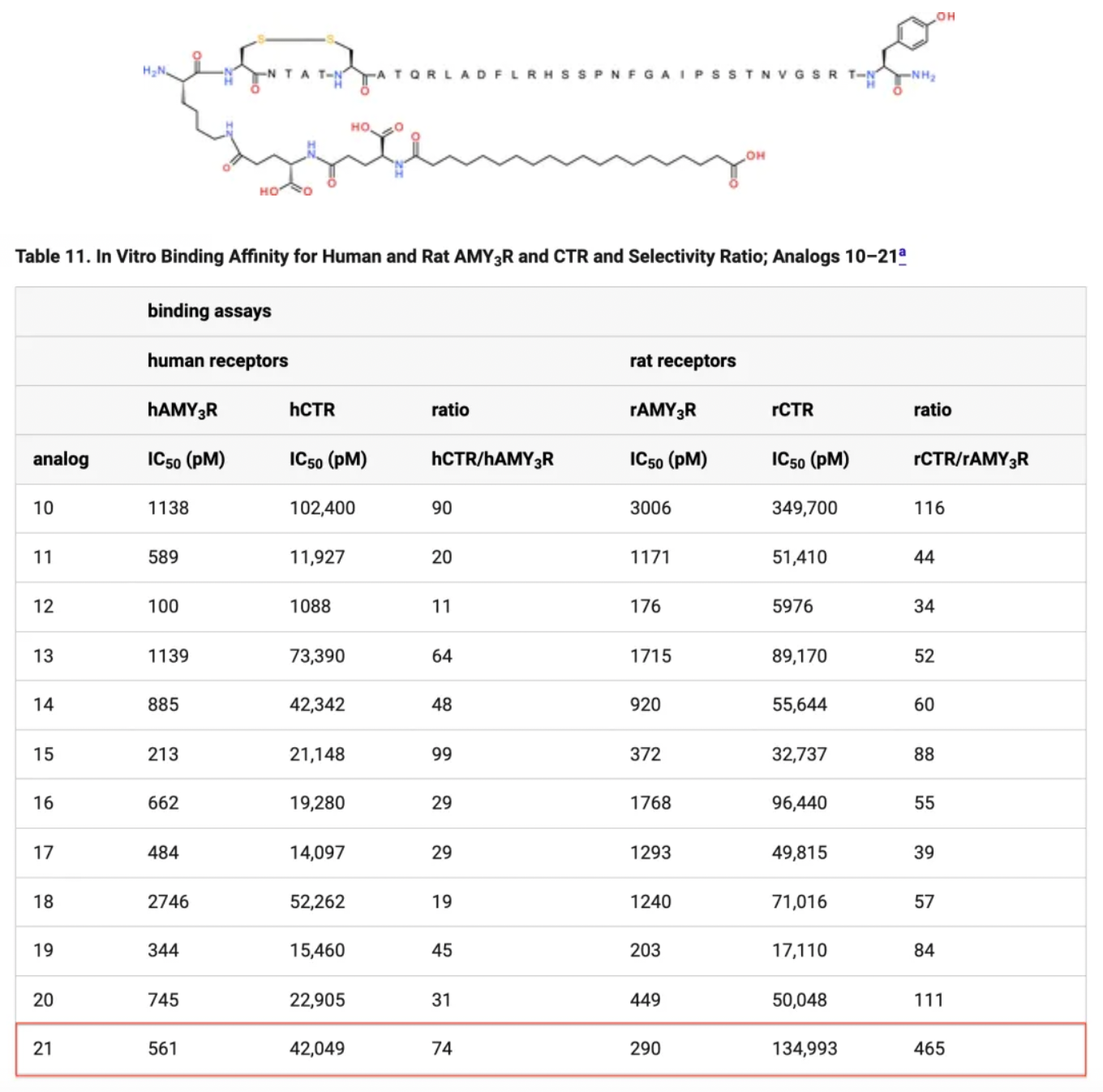
Researchers have explored a series of amino acid mutations and modifications, including replacing the 21st position with proline and various acylation modifications at different positions. In vivo peptide screening tested the ability of peptides to reduce appetite in rats via a single subcutaneous injection for up to 48 hours, with peptides 6, 11, 17, 20, and 21 showing sustained effects from 24 to 48 hours post-injection. When the isoelectric point (pI) is higher, the duration of action tends to shorten, possibly indicating reduced binding of the positively charged compounds with albumin. Peptide 21, due to its selectivity and prolonged effect, represents the best choice in this series, combining physical and chemical stability with AMYR selectivity and demonstrating promising in vivo efficacy.
Peptide 21, now referred to as NN1213, has been selected for further in vitro and in vivo characterization studies.
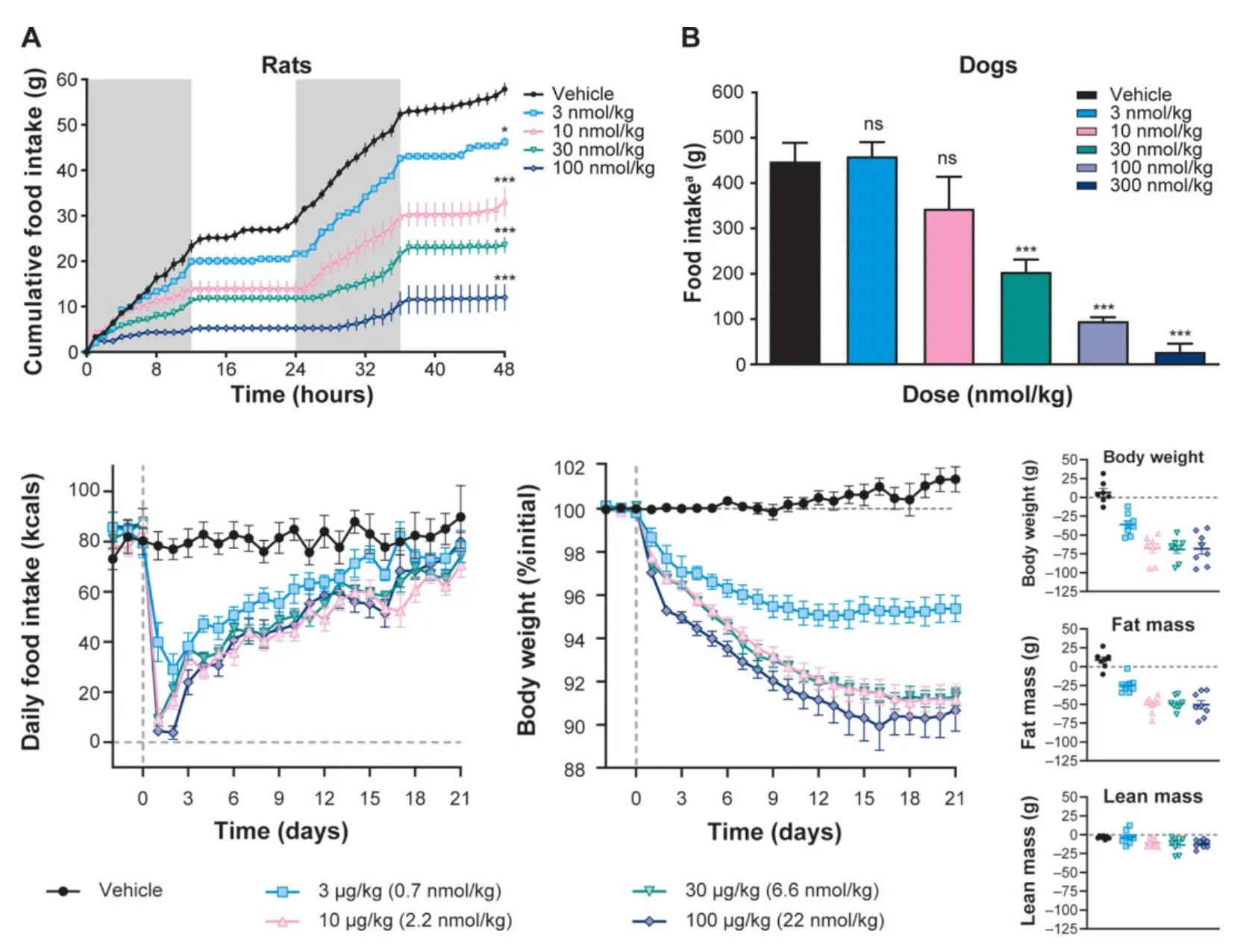
In rats and dogs, a single dose of NN1213 reduced appetite in a dose-dependent manner with prolonged effects. Consistent with the effects on appetite, studies in obese rats showed that daily administration of NN1213 led to a dose-dependent decrease in body weight over 21 days. MRI imaging revealed that this was primarily due to reduced fat mass, while lean mass remained largely unchanged.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!
Reference
https://pubs-acs-org.libproxy1.nus.edu.sg/doi/10.1021/acs.jmedchem.4c00022