Beacon Therapeutics Reports Positive 3-Month Phase 2 Results for XLRP Treatment
Beacon Therapeutics Holdings Limited, a prominent company specializing in ophthalmic gene therapy aimed at preserving and restoring vision for individuals suffering from severe retinal disorders, has announced the presentation of the interim safety and efficacy findings after three months of the Phase 2 DAWN trial in X-linked Retinitis Pigmentosa (XLRP) patients. This presentation took place at the FLORetina-ICOOR Meeting 2024 held in Florence, Italy.
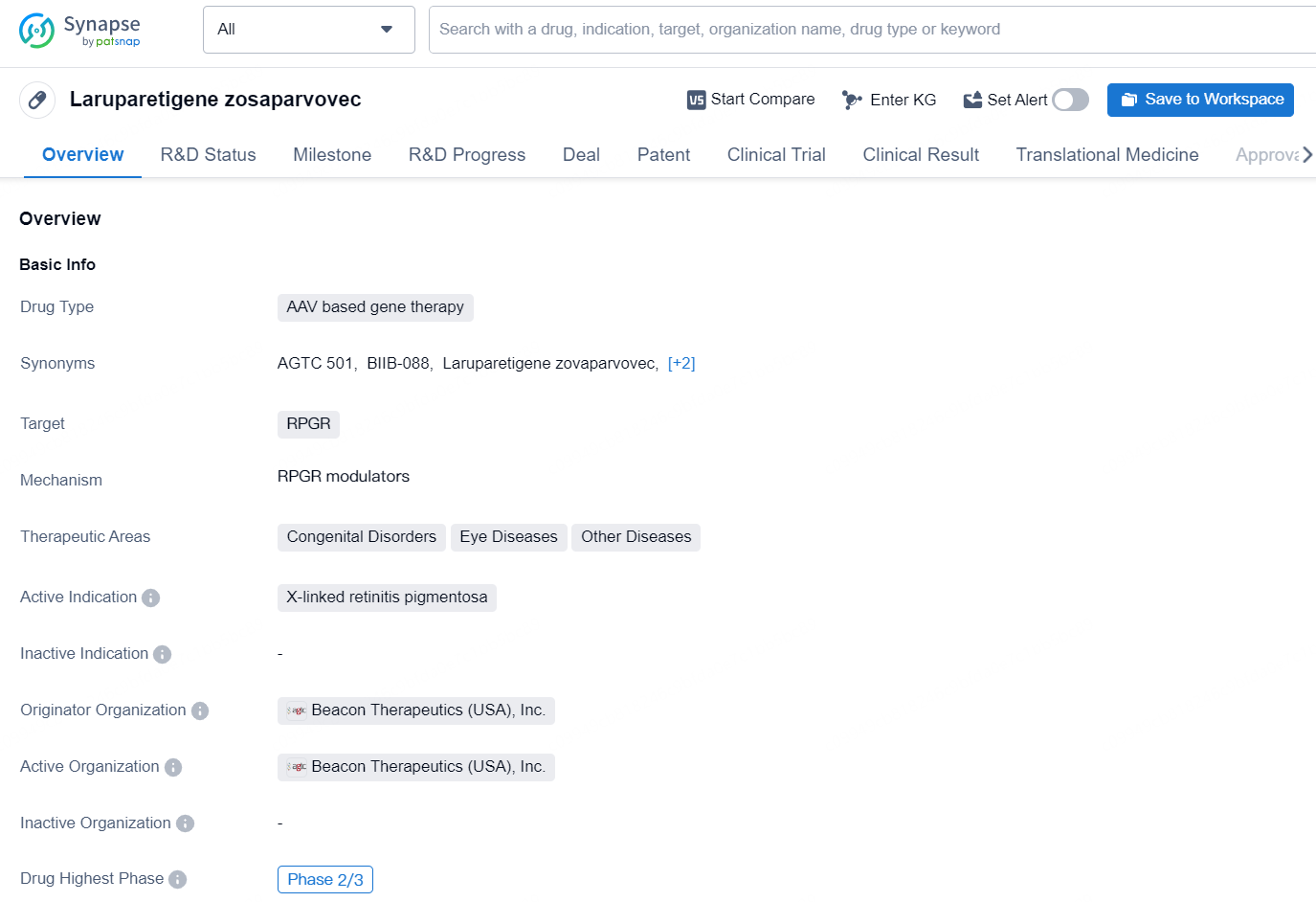
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The three-month findings indicate that laru-zova has been tolerated well among all participants. No treatment emergent adverse events (TEAEs) related to the study agent were reported, and there were no ocular inflammatory adverse events either. The data also suggest encouraging early enhancements in LLVA, an essential indicator of visual function. The benefit-risk profile is favorable for continuing clinical development aimed at treating patients with XLRP due to RPGR mutations.
DAWN is a non-randomized, open-label trial evaluating laru-zova in XLRP patients who have received prior treatment with a full-length AAV-vector based gene therapy targeting the RPGR protein. The aim of DAWN is to investigate the effectiveness, safety, and tolerability of two different doses of laru-zova in the untreated eye of participants who have undergone gene therapy for XLRP.
XLRP is a severe, inherited retinal disorder that commonly results in blindness by middle age, with no available treatment options. This condition predominantly affects young males, with an estimated incidence of 1 in 25,000 in the US, Europe, and Australia having XLRP caused by RPGR mutations. Laru-zova produces the complete RPGR protein, which is anticipated to alleviate the full extent of photoreceptor damage associated with XLRP, encompassing both rod and cone degeneration, thus representing a potentially leading treatment for progressive vision loss in these patients.
Lance Baldo, MD, CEO of Beacon Therapeutics, expressed optimism about the preliminary results from the Phase 2 DAWN trial, noting, “The robust safety profile observed so far is complemented by early promising enhancements in low luminance visual acuity, a significant measure of vision function in XLRP patients. These results not only support the ongoing pivotal VISTA trial but also reinforce our commitment to providing hope to those affected by this challenging disease.”
Additionally, Beacon Therapeutics is currently enrolling participants for its pivotal Phase 2/3 VISTA trial of laru-zova as it advances this potential treatment for XLRP patients.
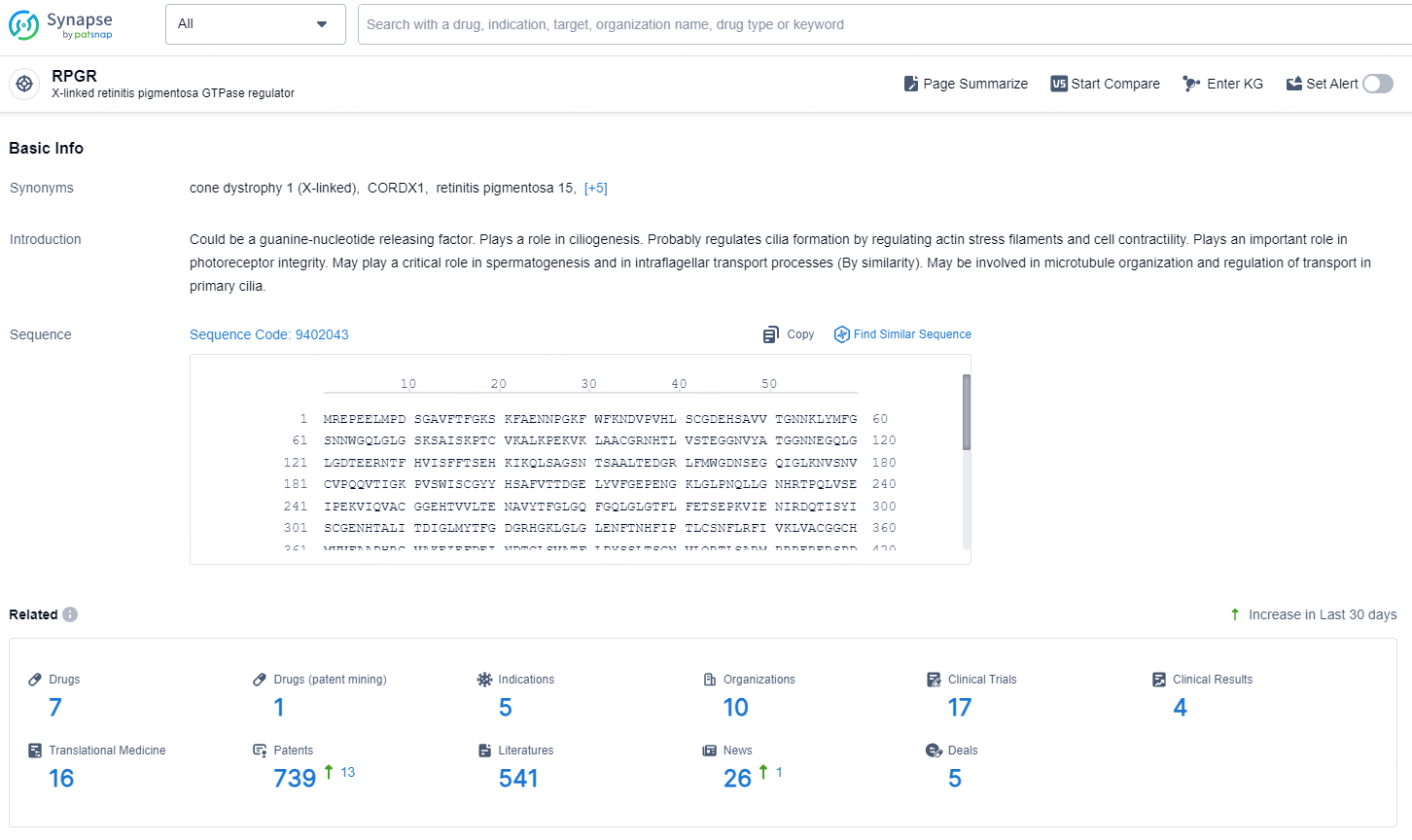
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of December 11, 2024, there are 7 investigational drugs for the RPGR target, including 5 indications, 10 R&D institutions involved, with related clinical trials reaching 17, and as many as 739 patents.
Laruparetigene zosaparvovec is an AAV-based gene therapy developed by Beacon Therapeutics (USA), Inc. The drug targets the RPGR gene and is intended for the treatment of X-linked retinitis pigmentosa, a genetic eye disorder that causes vision loss. In addition to eye diseases, laruparetigene zosaparvovec also has potential applications for congenital disorders and other diseases.