IMFINZI® Granted Priority Review in U.S. for Muscle-Invasive Bladder Cancer
AstraZeneca's supplemental Biologics License Application (sBLA) for IMFINZI®(durvalumab) has received acceptance and been awarded Priority Review status in the United States for treating individuals with muscle-invasive bladder cancer (MIBC).
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The Food and Drug Administration (FDA) has designated Priority Review for drug applications that, upon approval, promise considerable advancements over existing treatments by proving enhancements in safety or efficacy, halting critical conditions, or improving patient adherence. The FDA action date, as stated in the Prescription Drug User Fee Act, is expected in the second quarter of 2025.
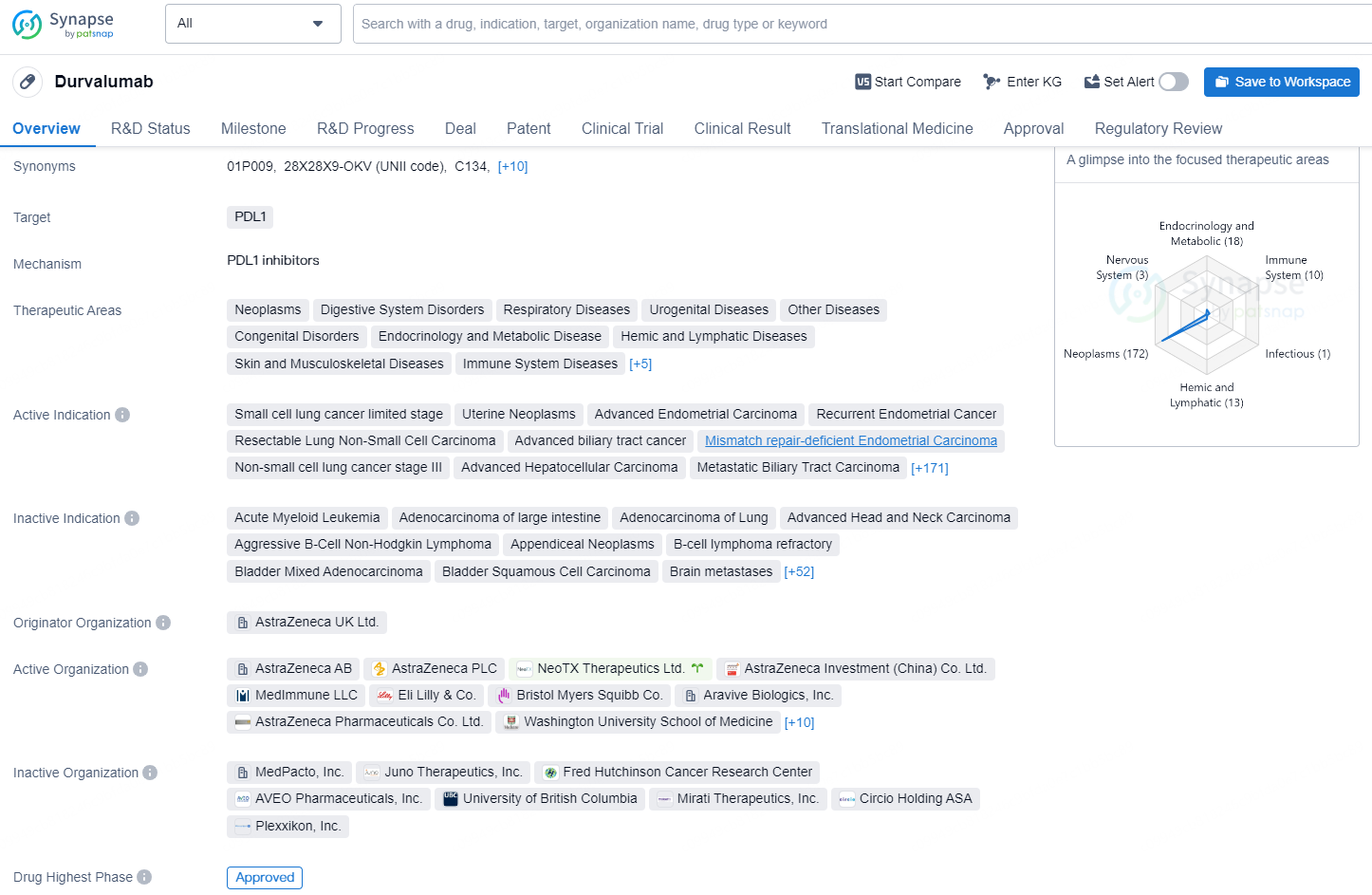
Around 25% of individuals diagnosed with bladder cancer present with tumors that invade the bladder's muscular wall (without metastasizing), known as muscle-invasive bladder cancer (MIBC). In MIBC, which is a curative-intent scenario, about 117,000 patients are currently receiving the standard treatment, which comprises neoadjuvant chemotherapy followed by radical cystectomy. Nonetheless, even after undergoing cystectomy, there is a considerable risk of disease recurrence and a grim outlook for these patients.
Susan Galbraith, Executive Vice President of Oncology R&D at AstraZeneca, stated: “There is an essential need for new therapies targeting muscle-invasive bladder cancer because nearly half of the patients will experience either a return of their cancer or its progression despite curative efforts, including complete bladder removal. The current Priority Review status acknowledges the pressing requirement for innovative solutions for these patients and indicates the potential of IMFINZI to elevate the existing standard care as the first and only perioperative immunotherapy regimen aimed at delaying recurrence and improving survival rates in this patient population.”
The supplemental Biologics License Application (sBLA) is founded on results from the NIAGARA Phase III clinical trial, which were presented at the Presidential Symposium during the 2024 European Society for Medical Oncology (ESMO) Congress and simultaneously published in The New England Journal of Medicine.
Within the trial, participants received IMFINZI alongside neoadjuvant chemotherapy prior to radical cystectomy, followed by IMFINZI as post-surgery monotherapy, or they underwent only neoadjuvant chemotherapy followed by radical cystectomy. An interim analysis indicated that perioperative IMFINZI led to a 32% decrease in the risk of disease progression, recurrence, surgery avoidance, or mortality compared to standard neoadjuvant chemotherapy followed by radical cystectomy alone (reflected in the event-free survival [EFS] hazard ratio [HR] of 0.68; 95% confidence interval [CI] 0.56-0.82; p<0.0001). The estimated median EFS was not yet achieved in the IMFINZI group, while the comparator group had an EFS of 46.1 months. Approximately 67.8% of patients on the IMFINZI regimen remained event-free after two years, compared to 59.8% in the comparator group.
Results related to the critical secondary endpoint of overall survival (OS) demonstrated that the IMFINZI perioperative regimen lowered the mortality risk by 25% in comparison to neoadjuvant chemotherapy followed by radical cystectomy (indicated by OS HR of 0.75; 95% CI 0.59-0.93; p=0.0106). Median survival had not yet been reached in either treatment group. An estimated 82.2% of patients on the IMFINZI protocol were alive two years post-treatment, as opposed to 75.2% in the comparator cohort.
IMFINZI exhibited a favorable tolerance profile, with no novel safety concerns arising from its use in neoadjuvant and adjuvant contexts. Additionally, the integration of IMFINZI with neoadjuvant chemotherapy aligned with known safety profiles of the respective drugs and did not affect patients' ability to complete four cycles of chemotherapy or proceed to surgery, in contrast to receiving neoadjuvant chemotherapy alone.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
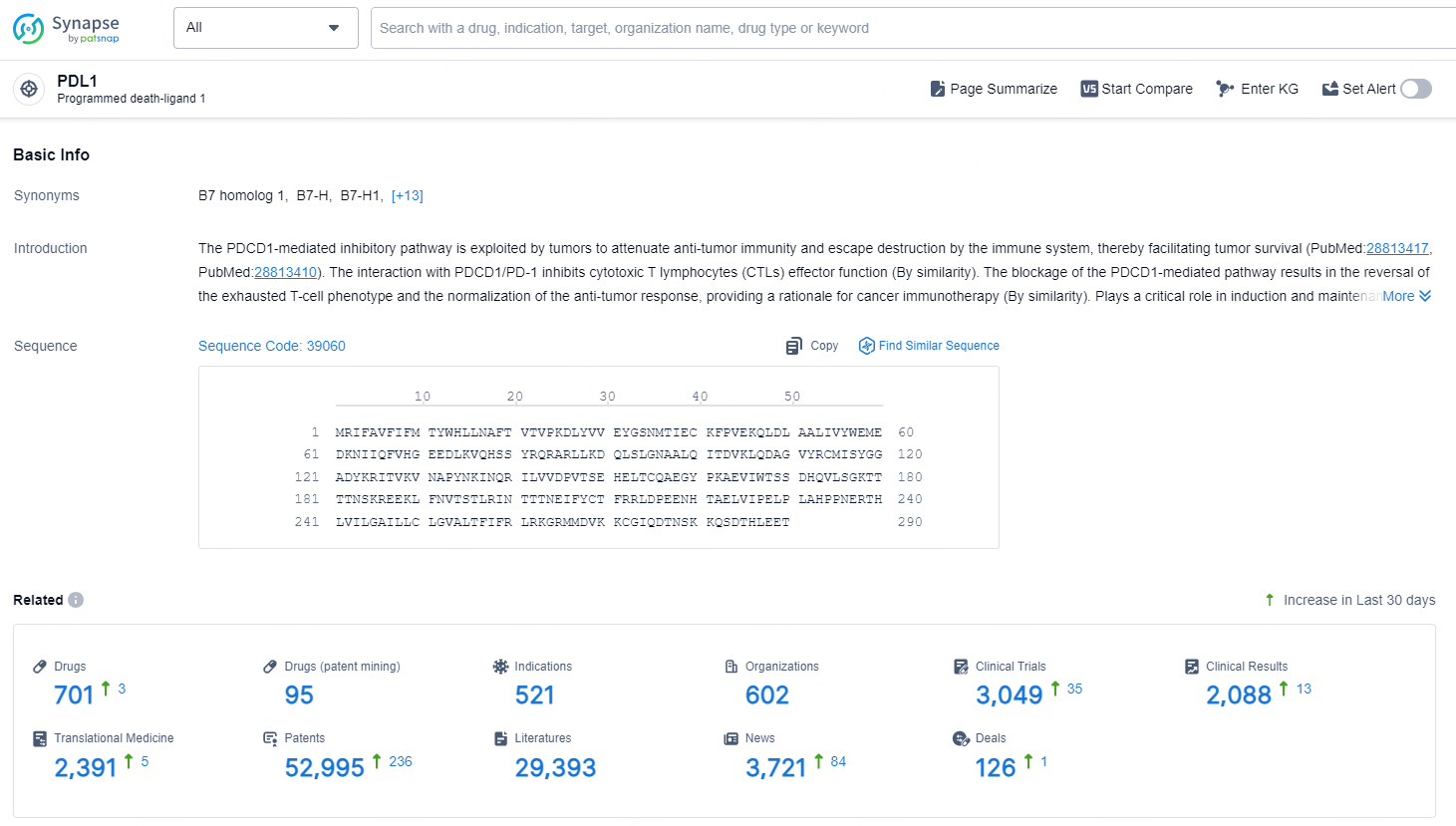
According to the data provided by the Synapse Database, As of December 11, 2024, there are 701 investigational drugs for the PDL1 target, including 521 indications, 602 R&D institutions involved, with related clinical trials reaching 3049, and as many as 52995 patents.
Durvalumab is a drug that belongs to the class of monoclonal antibodies and functions as a programmed death-ligand 1 (PDL1) inhibitor. Its primary indication is for the treatment of advanced endometrial carcinoma. The drug was first approved in the United States on August 15, 2024. By inhibiting PDL1, Durvalumab can enhance the body's immune response against cancer cells, as PDL1 is a ligand that, when bound to its receptor, can suppress the immune system's ability to attack tumor cells.