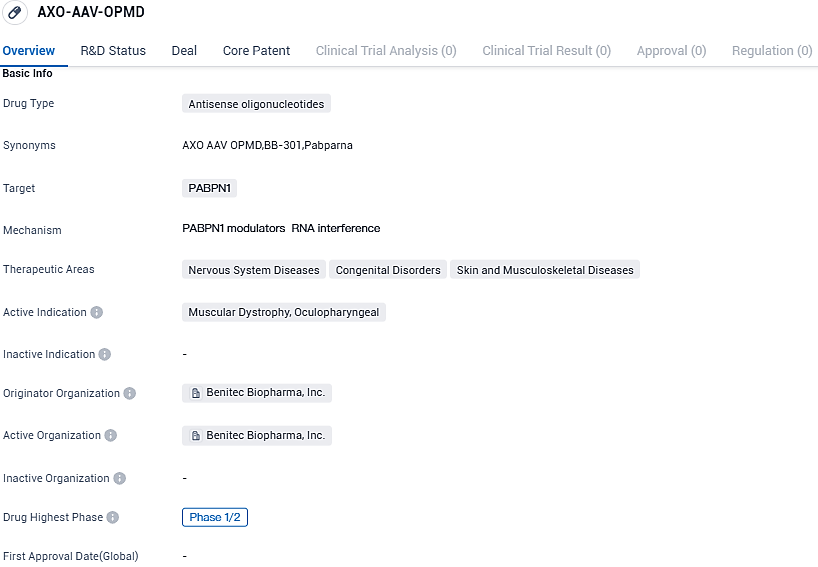
Benitec Biopharma begins early trial dosing for BB-301, its genetic therapy for Oculopharyngeal Muscular Dystrophy
Benitec Biopharma Inc., an enterprise at the clinical phase specializing in the realm of gene therapy, which is formulating innovative genetic treatments with the aid of its unique DNA-directed RNA interference technology, known as the "Silence and Replace" platform, has revealed that initial dosing has commenced with a participant in the early-to-mid-stage BB-301 clinical investigation.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
As the first investigational gene therapy employing the Silence and Replace strategy, BB-301 is in development by the firm to address Dysphagia caused by Oculopharyngeal Muscular Dystrophy.
The commencement of the initial participant in the trial signals the start of a one-year observation phase, crafted to enable the definitive assessment of both primary and secondary objectives in the BB-301 Phase 1b/2a Clinical Study. Safety and efficacy will be periodically assessed after every 90-day interval post the application of BB-301.
Jerel A. Banks, M.D., Ph.D., Executive Chairman and CEO of Benitec expressed, “It's a privilege to begin the clinical examination of BB-301, and we are buoyed by the strides in research and development thus far. Forging ahead into the clinical affirmation of our gene therapy approach based on Silence and Replace holds promise for those affected by genetically identifiable conditions. Our unwavering mission is to enhance the lives of those living with OPMD.”
Oculopharyngeal Muscular Dystrophy represents a severe, genetic pathology that affects about 15,000 individuals across the U.S., Canada, Western Europe, and Israel. It progressively robs patients of their capacity to ingest food and drink, leading to persistent undernourishment, risk of aspiration, and potentially lethal aspiration pneumonia episodes. To date, no treatments have received approval to combat OPMD.
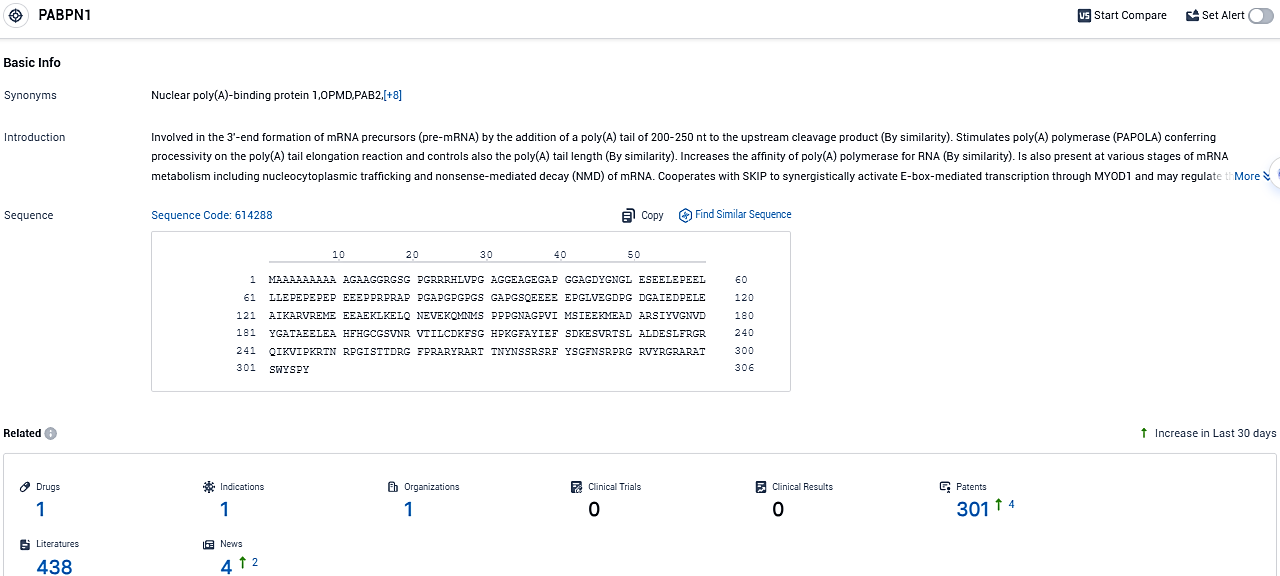
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 7, 2023, there are 1 investigational drugs for the PABPN1 target, including 1 indications, 1 R&D institutions involved, and as many as 301 patents.
BB-301 is a novel, modified AAV9 capsid expressing a unique, single bifunctional construct promoting co-expression of both codon-optimized Poly-A Binding Protein Nuclear-1 (PABPN1) and two small inhibitory RNAs against mutant PABPN1. While the drug shows promise in addressing the symptoms of OPMD, more research is needed to fully understand its potential benefits and risks.