Grünenthal acquires Valinor for $250 million to obtain an opioid antagonist product
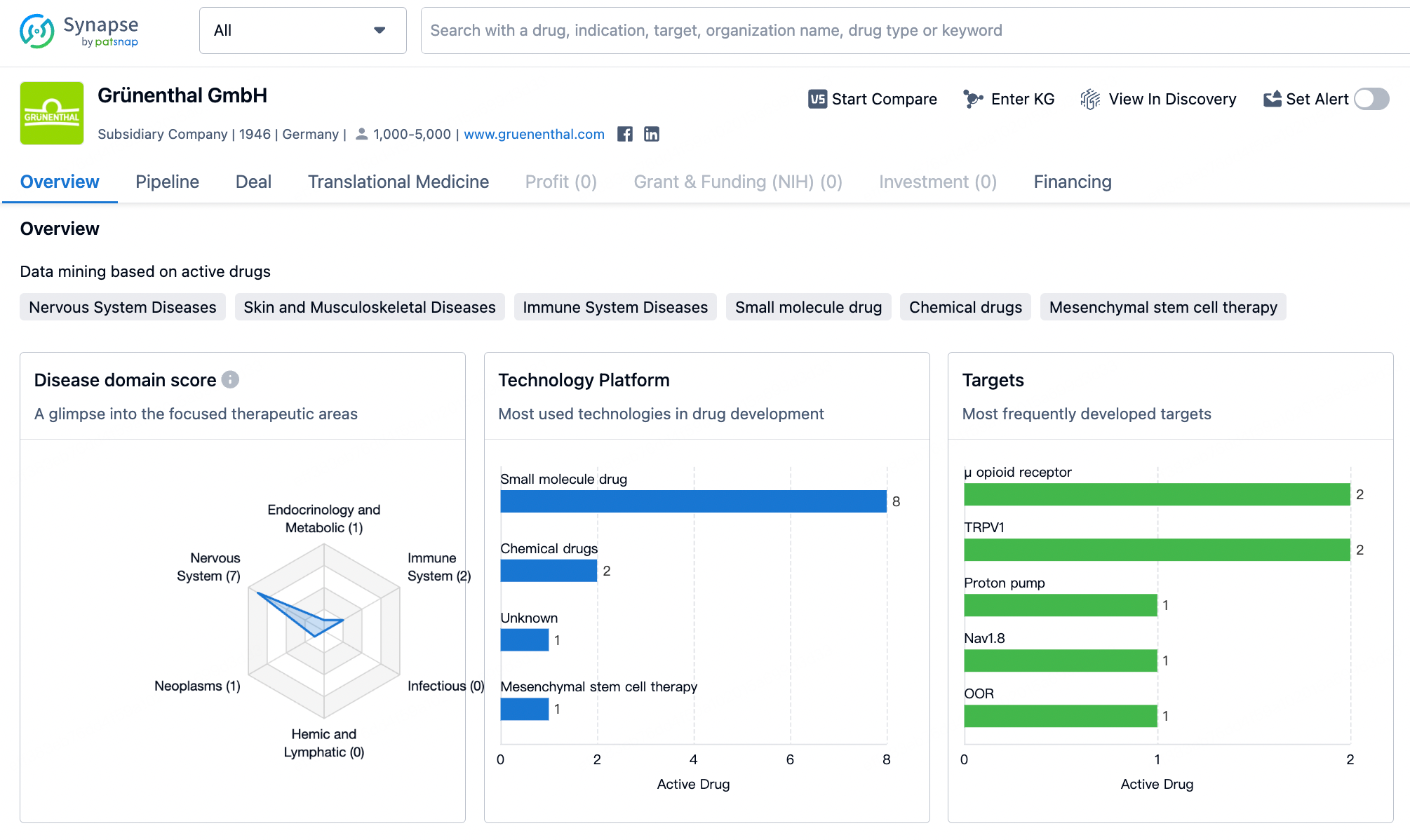
Recently, the German company Grünenthal, specializing in the development of pain relief medications, announced the acquisition of the American pharmaceutical manufacturer Valinor Pharma for $250 million in order to obtain the rights to Movantik (naloxegol), a treatment for opioid-induced constipation developed by Valinor.
It is reported that in 2023, sales of this product reached $200 million in the United States. Since Grünenthal already holds the rights to Movantik in the European Union, the acquisition of Valinor will allow Grünenthal to have complete control over the Movantik brand in all markets outside of Canada. The United States is considered the company's most important growth market, and the acquisition of Valinor will strengthen Grünenthal's position in the pain management sector in the U.S.
About Naloxegol
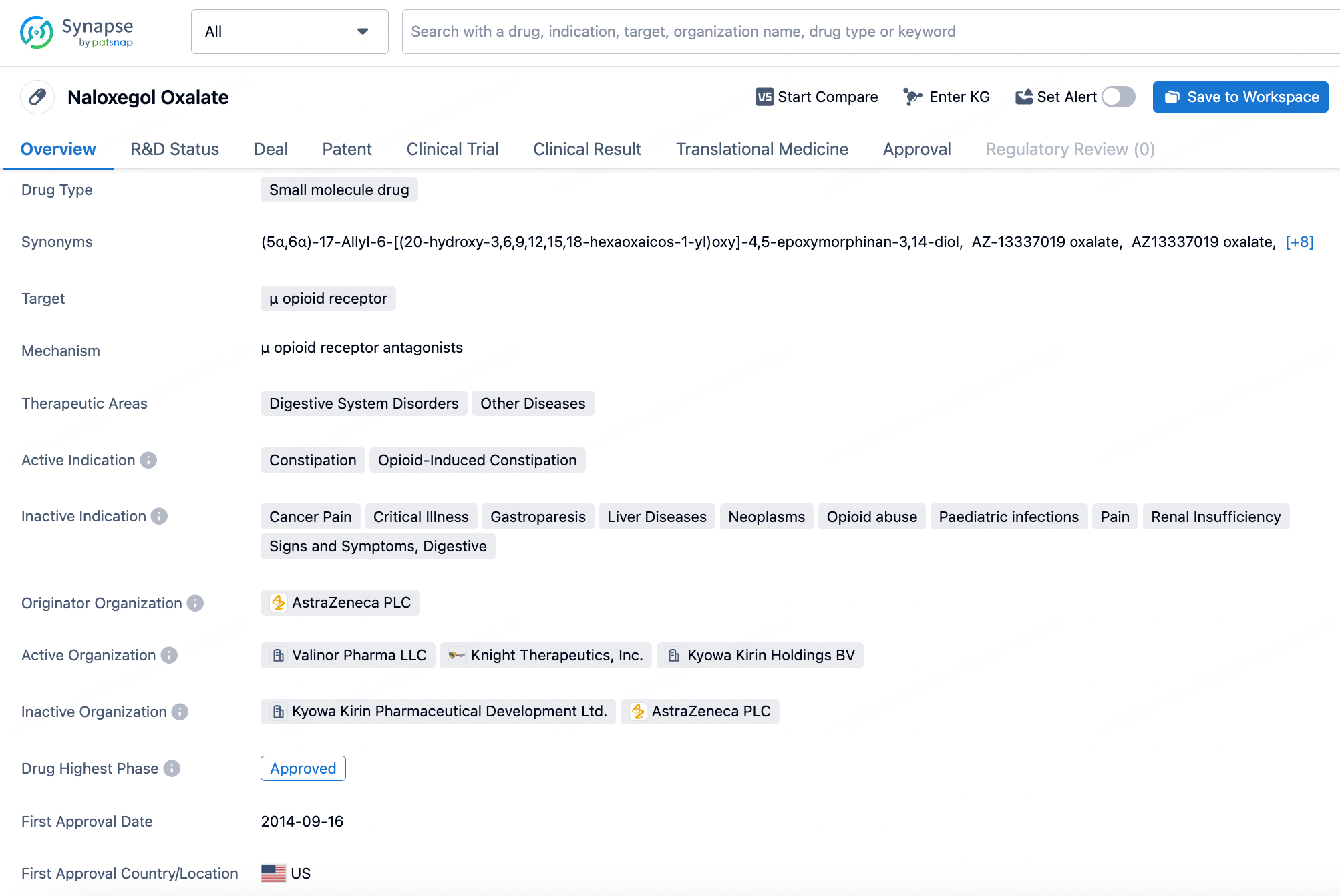
Naloxegol is a selective peripherally-acting μ-opioid receptor antagonist (PAMORA) and a pegylated form of naloxone. This product was originally developed by Nektar Therapeutics and further developed by AstraZeneca. It was approved by the U.S. FDA in September 2014 for the treatment of opioid-induced constipation in adults with chronic non-cancer pain. Notably, Nektar holds a patent for its drug pegylation technology, and naloxegol's advantage over the opioid receptor antagonist naloxone stems from its pegylated structure, which confers high selectivity for peripheral opioid receptors while not crossing the blood-brain barrier.
According to a pharmacological study published in 2017, naloxegol is a potent antagonist at human μ-opioid receptors in vitro, with a Ki value of 7.42 nM, demonstrating an action similar to that of non-PEGylated naloxone.
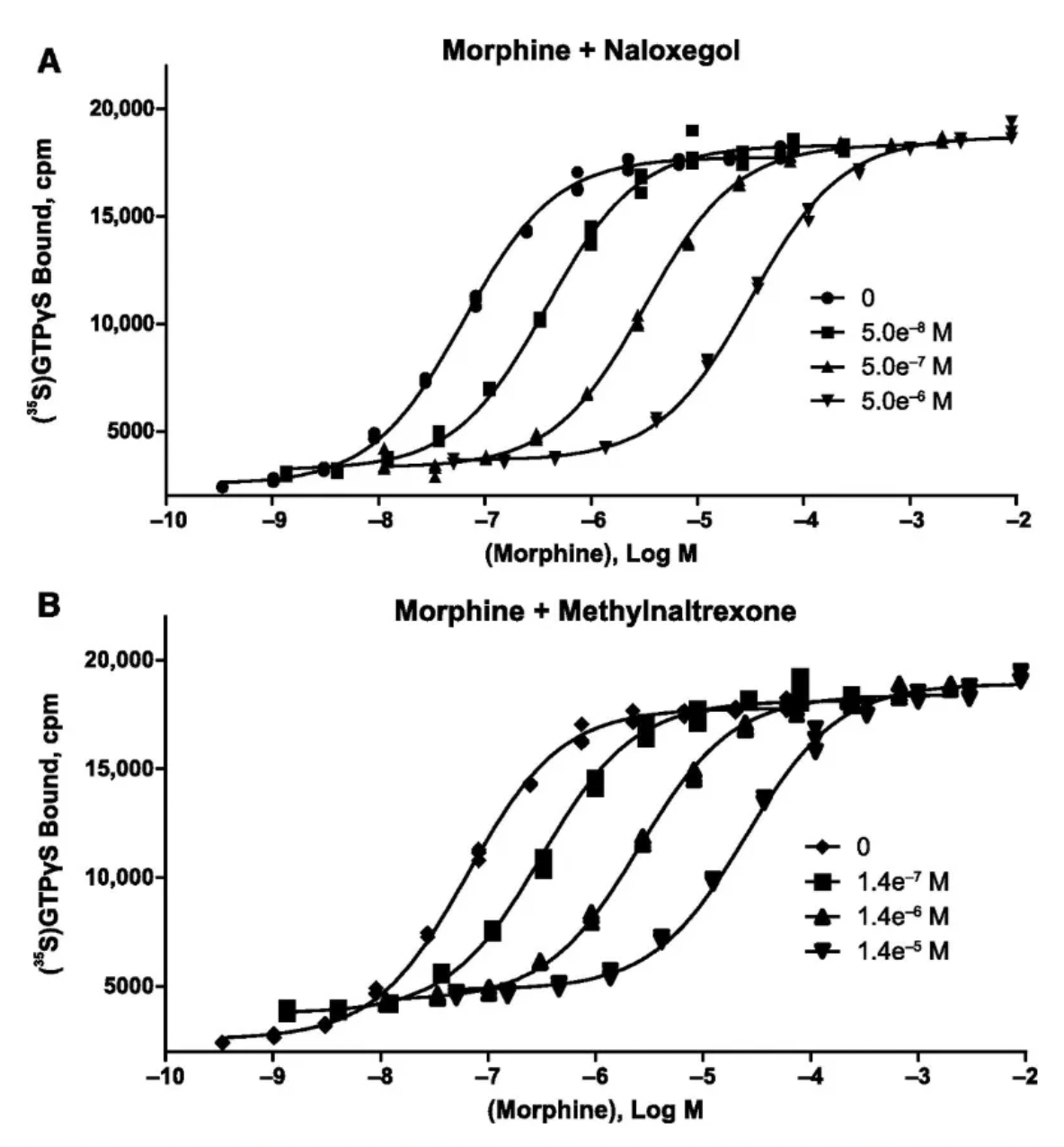
AstraZeneca published the clinical study results of Naloxegol in the New England Journal of Medicine in 2014. Compared to placebo, the use of Naloxegol significantly improved treatment response rates without reducing the analgesic effects mediated by opioids.

In addition to Naloxegol, Nektar developed ADYNOVATE in collaboration with Baxter and Baxalta. ADYNOVATE is an extended half-life recombinant Factor VIII (rFVIII) product for the treatment of hemophilia. In January 2019, Takeda Pharmaceutical acquired Shire, gaining ownership of Baxalta and ADYNOVATE as a result.
About PEGylated Pharmaceuticals
PEGylated pharmaceuticals are composed of various reagents, including linkers, crosslinkers, and labels. Different types of reagents include amine, carboxyl, and carboxyl PEGylation. Currently, PEGylated drugs are primarily used to treat conditions such as cancer, chronic kidney disease, hepatitis, multiple sclerosis, hemophilia, and gastrointestinal diseases.
In March 1990, ADAGEN produced by Enzon Pharmaceuticals was the first PEGylated protein approved by the U.S. Food and Drug Administration (FDA) for market entry. Since the launch of ADAGEN, a large number of PEGylated proteins and peptide drugs have followed suit, and many more are in clinical trials or development stages. Among the FDA-approved drugs, Pegasys and Neulasta generated sales exceeding $5 billion in 2011.
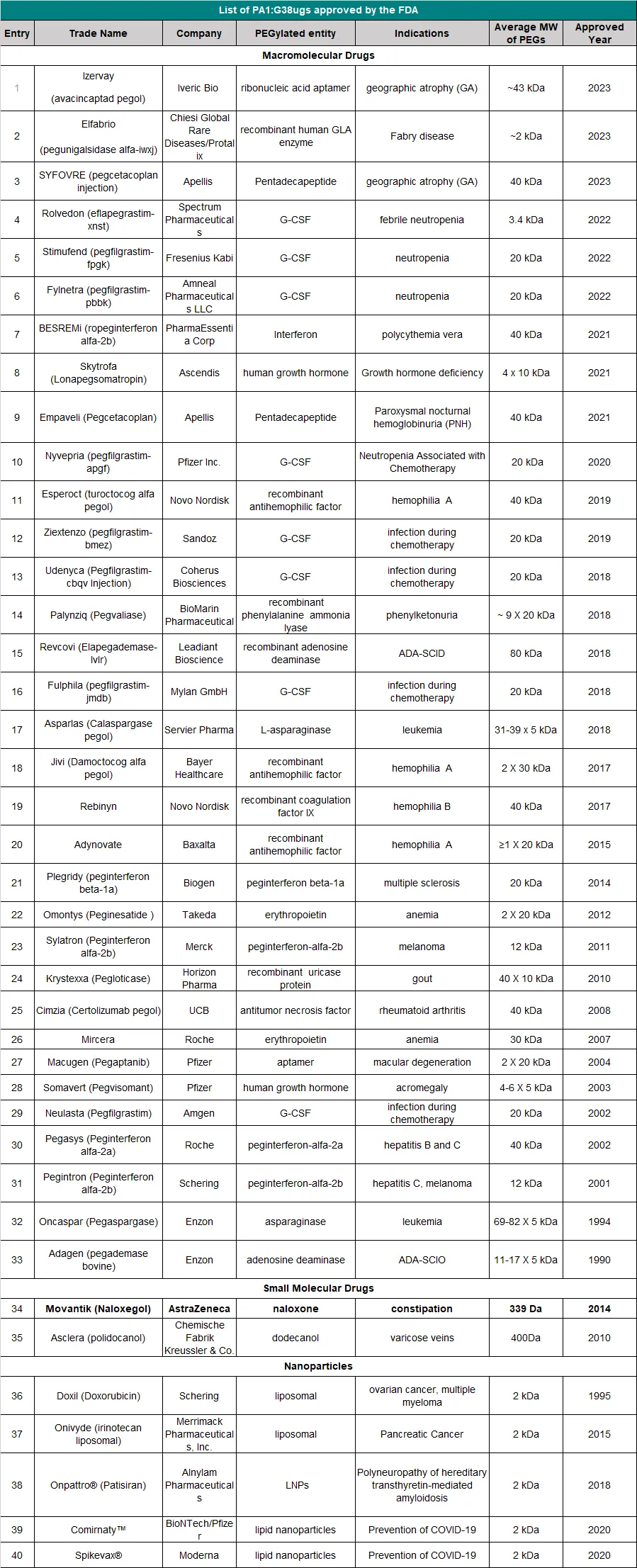
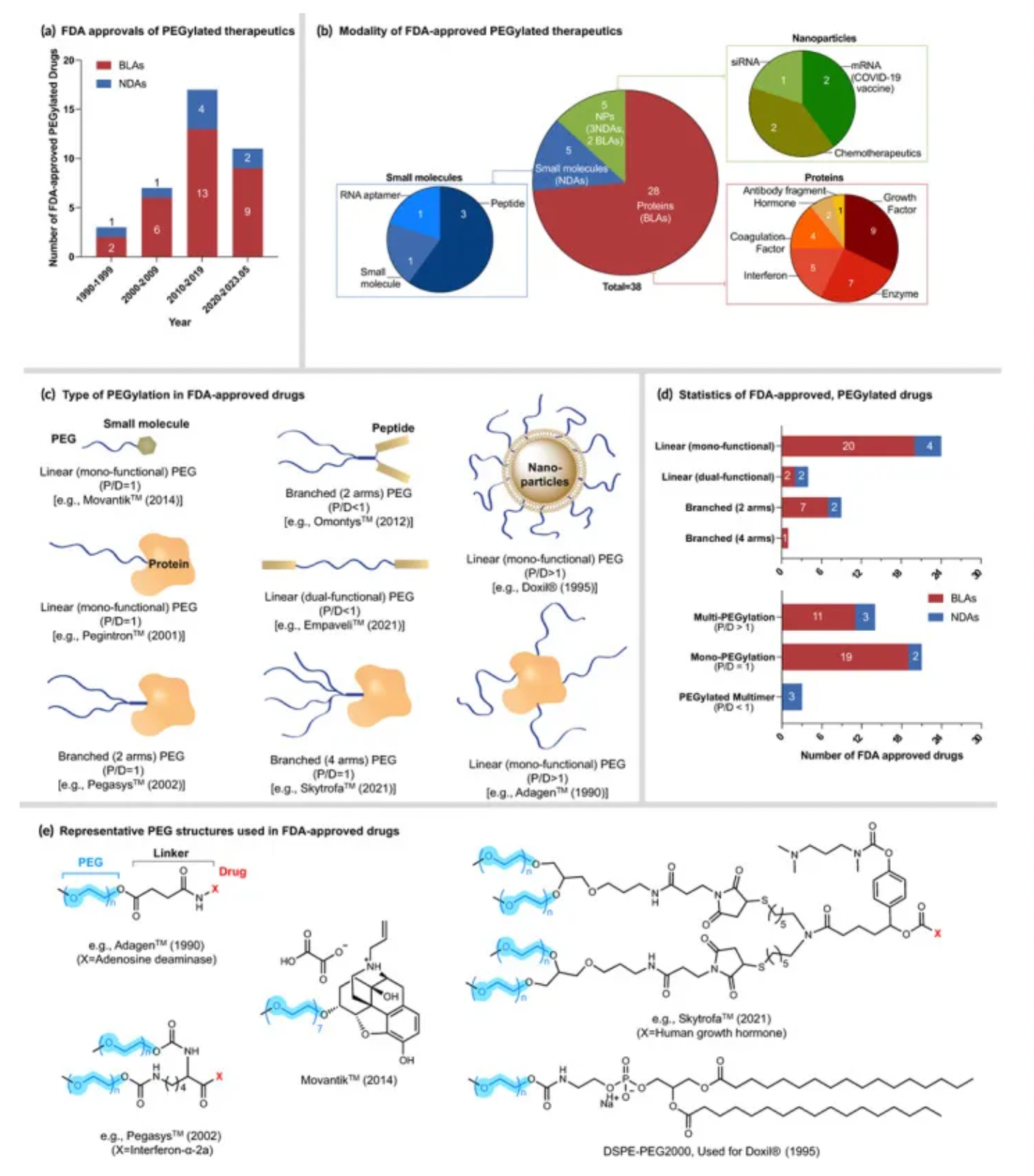
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!