Biohaven Announces Positive Results in Key Study of Troriluzole for Spinocerebellar Ataxia (SCA)
Biohaven Ltd. (NYSE: BHVN) (Biohaven or the Company) announced that pivotal Study BHV4157-206-RWE (NCT06529146) yielded positive topline results. These results indicate the effectiveness of troriluzole in changing the f-SARA score from baseline after three years of therapy. The study successfully met its primary endpoint, showing statistically significant improvements in the f-SARA at the end of the first and second years. SCA, a rare and progressively worsening neurodegenerative disorder, impacts around 15,000 individuals in the United States and 24,000 in Europe and the United Kingdom. Currently, there are no FDA-approved treatments for SCA.
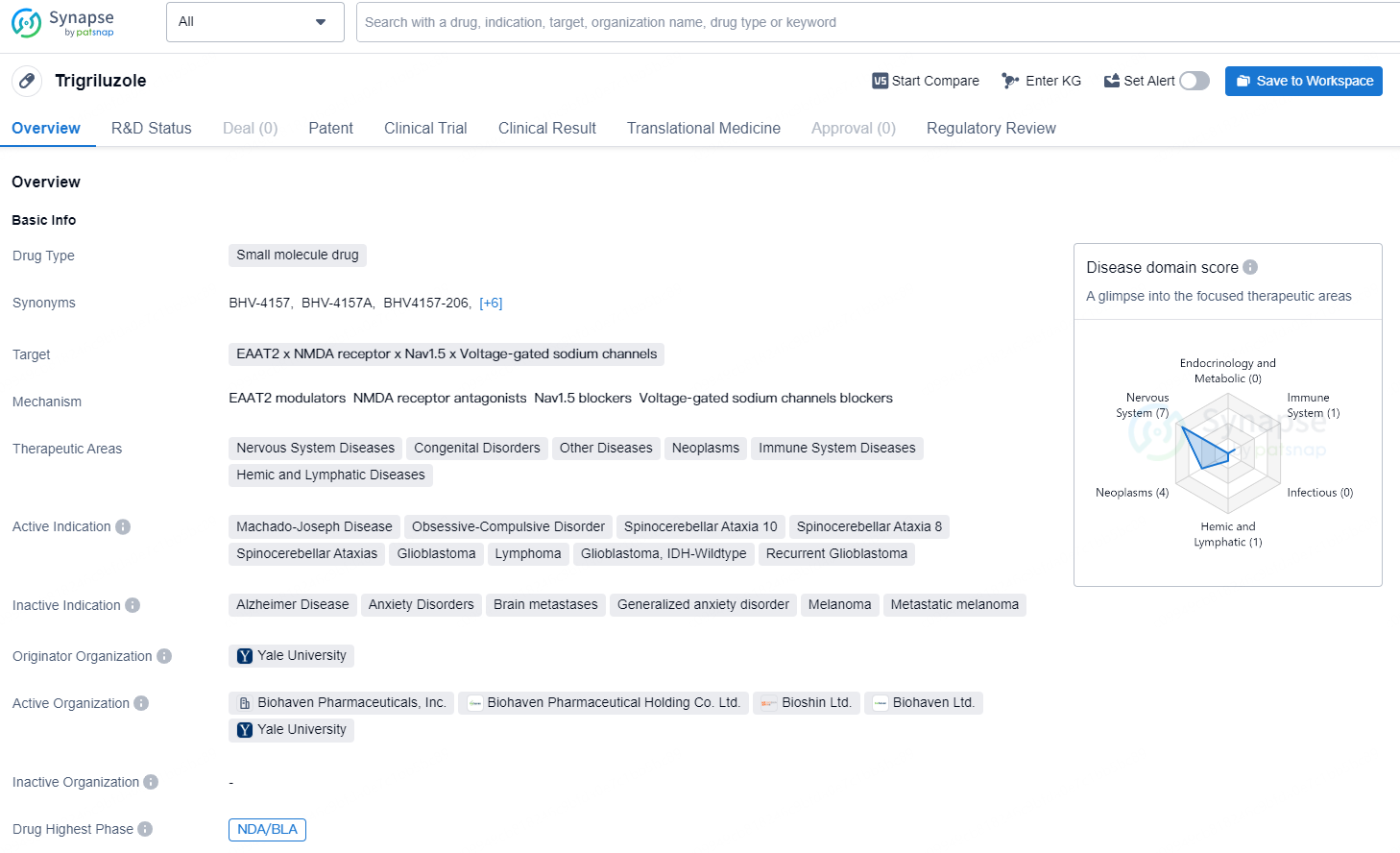
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Collectively, evidence from various analyses indicates a significant and clinically relevant deceleration of disease progression in SCA patients. These therapeutic advantages result in a 50-70% slower decline rate compared to those untreated, equating to a 1.5-2.2 year delay in disease progression over a 3-year study duration. Furthermore, a responder sensitivity analysis, delineating disease progression by a 2-point or greater worsening on the f-SARA over 3 years, revealed an odds ratio (OR) of 4.1 (95% CI: 2.1, 8.1) for the untreated external control group compared to troriluzole-treated individuals (p < 0.0001; pooled analysis).
Dr. Susan Perlman, Head of Ataxia Clinic and Neurogenetics Clinical Trials at the David Geffen School of Medicine at UCLA, commented, “SCA is a severe, progressively degenerative disorder that severely impairs quality of life, leaving patients incapable of performing self-care, walking, or speaking. Troriluzole is the first treatment to exhibit a reduction in disease progression, potentially granting patients extra years of independence, during which they can walk unassisted, continue working, engage with their children, and partake in daily activities. This represents an encouraging and hopeful development for the SCA community.”
Study BHV4157-206-RWE, developed in collaboration with the US Food and Drug Administration (FDA), aimed to evaluate the efficacy of troriluzole in SCA over 3 years, gauged by the change in f-SARA scores from baseline. The study incorporated Phase 3 data and an external control of matched, untreated SCA subjects from the US Clinical Research Consortium for the Study of Cerebellar Ataxia (CRC-SCA), adhering to the FDA’s Guidance on Real-World Evidence (RWE) of effectiveness. All endpoints were pre-established, and both the study protocol and statistical analysis plan were submitted to and reviewed by the FDA before the topline data analysis.
This new analysis doubled the previous 3-year dataset, with 63 participants now completing 3 years of troriluzole treatment and matched to the control arm. Propensity Score Matching (PSM) ensured that untreated patients from the CRC-SCA study were rigorously matched to treated individuals from Study BHV4157-206 based on baseline characteristics. The primary goal was to examine the effects of troriluzole treatment for up to 3 years by comparing f-SARA data from treated patients in Study BHV4157-206 to untreated individuals in the natural history study. Troriluzole-treated patients showed statistically significant and lasting benefits at 1, 2, and 3 years on the f-SARA compared to a rigorously matched natural history control.
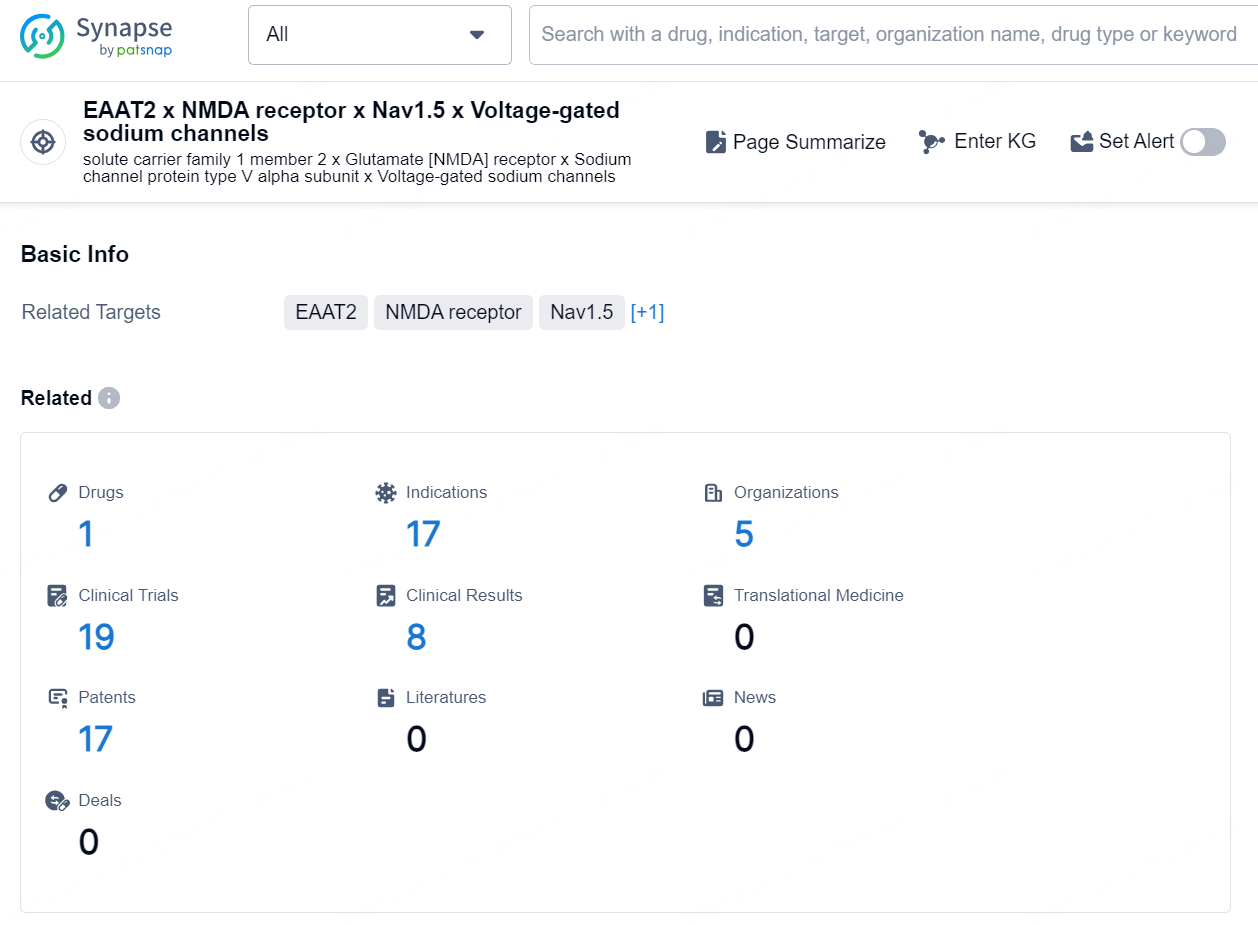
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 24, 2024, there are 1 investigational drug for the EAAT2, NMDA receptor, Nav1.5, and voltage-gated sodium channels target, including 17 indications, 5 R&D institutions involved, with related clinical trials reaching 19, and as many as 17 patents.
Trigriluzole is a small molecule drug that targets EAAT2, NMDA receptor, Nav1.5, and voltage-gated sodium channels. It is primarily focused on treating a range of conditions within various therapeutic areas including nervous system diseases, congenital disorders, other diseases, neoplasms, immune system diseases, and hemic and lymphatic diseases. The active indications for Trigriluzole include Machado-Joseph Disease, Obsessive-Compulsive Disorder, Spinocerebellar Ataxia 10, Spinocerebellar Ataxia 8, Spinocerebellar Ataxias, Glioblastoma, Lymphoma, and Recurrent Glioblastoma. Trigriluzole is originated from Yale University, and it has reached the highest global phase of NDA/BLA, and the highest phase in China is Phase 3.