BioNova Pharmaceuticals' oral Menin inhibitor BN104 has received Fast Track designation from the FDA for the treatment of acute myeloid leukemia
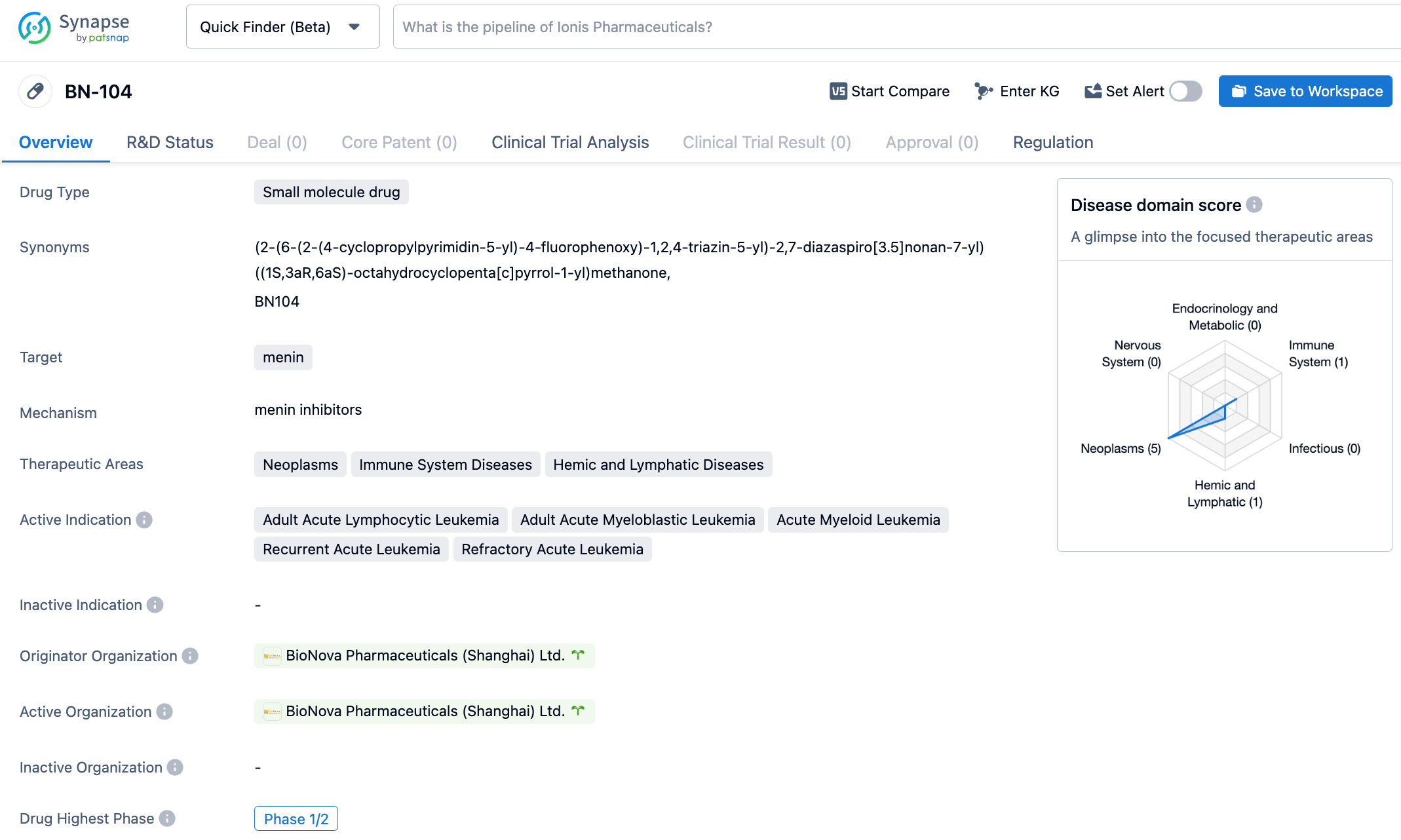
On October 24, 2023, BioNova Pharmaceuticals announced that the U.S. FDA has granted Fast Track Designation to its product BN104 for the treatment of relapsed/refractory acute leukemia. BN104 is a novel and highly selective oral Menin inhibitor developed by BioNova Pharmaceuticals. It has previously been approved for clinical research in the United States and has been granted Orphan Drug Designation by the FDA for the treatment of acute myeloid leukemia (AML).
BN104 is a novel and highly selective oral Menin inhibitor designed and discovered by BioNova Pharmaceuticals. Menin inhibitors are currently considered by the academic community to be a potential treatment for global acute leukemia patients carrying MLL gene rearrangements or NPM1 gene mutations. In preclinical research compared with other clinical-stage Menin inhibitors, BN104 has shown superior efficacy and a significantly larger safety window. In April 2023, BN104 received Orphan Drug Designation from the FDA. In August and September 2023, BN104 received Investigational New Drug permissions in China and the United States respectively. On October 19, 2023, BN104 completed its first patient administration in China.
Menin, encoded by the MEN1 gene, mainly exists in the nucleus of cells, serving multiple key roles in biological pathways such as cellular growth regulation, cell cycle control, genomic stability, bone development, and hematopoiesis as a scaffolding protein. It interacts with different binding partners in the nucleus to regulate gene transcription and interacts with various signaling pathways. Research has discovered that Menin inhibitors are effective against leukemia with KMT2A rearrangement (KMT2Ar) and NPM1 mutation (NPM1c), which account for approximately 5-10% and 20-30% of patients with acute lymphoblastic leukemia (ALL) and AML respectively.
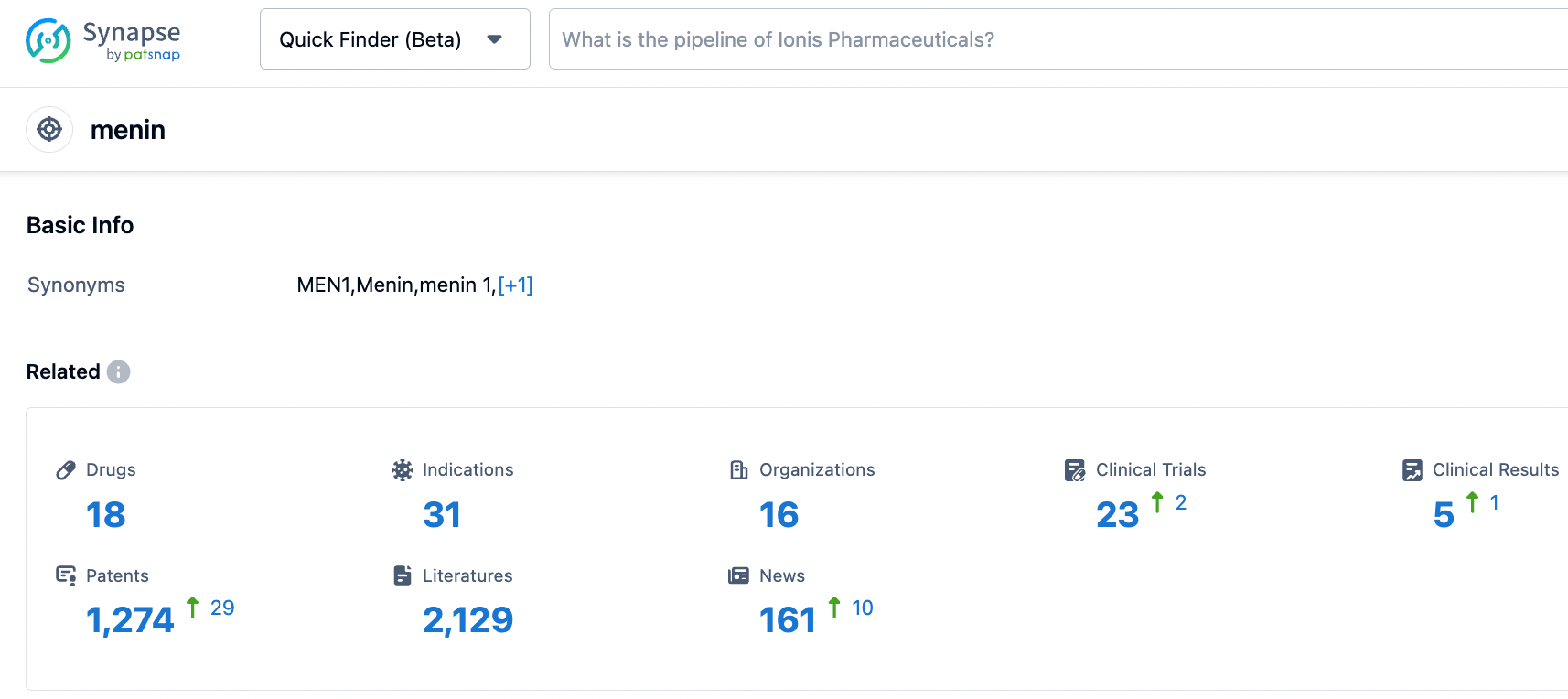
According to information disclosed by the Synapse database, as of October 25, 2023, there are a total of 18 investigational drugs targeting Menin, covering 31 indications, involving 16 research institutions, and 23 related clinical trials, with as many as 1274 patents. Acute myeloid leukemia is a blood cancer with generally poor prognosis, where current treatments include chemotherapy and stem cell transplantation. Despite some effective targeted therapies, treatments for patients with KMT2A gene rearrangement and NPM1 mutation subtype are still limited. Menin inhibitors, as a new drug for the treatment of this subtype, have shown promising treatment potential in early clinical trials. Several pharmaceutical companies are already positioning themselves in this market, with the furthest advanced in phase two clinical development. It is anticipated that these inhibitors will be approved soon, providing hope for patients.