Biotheryx Receives FDA Approval to Begin Trials for BTX-9341, a Novel Dual-Action Degrader Targeting CDK4/6
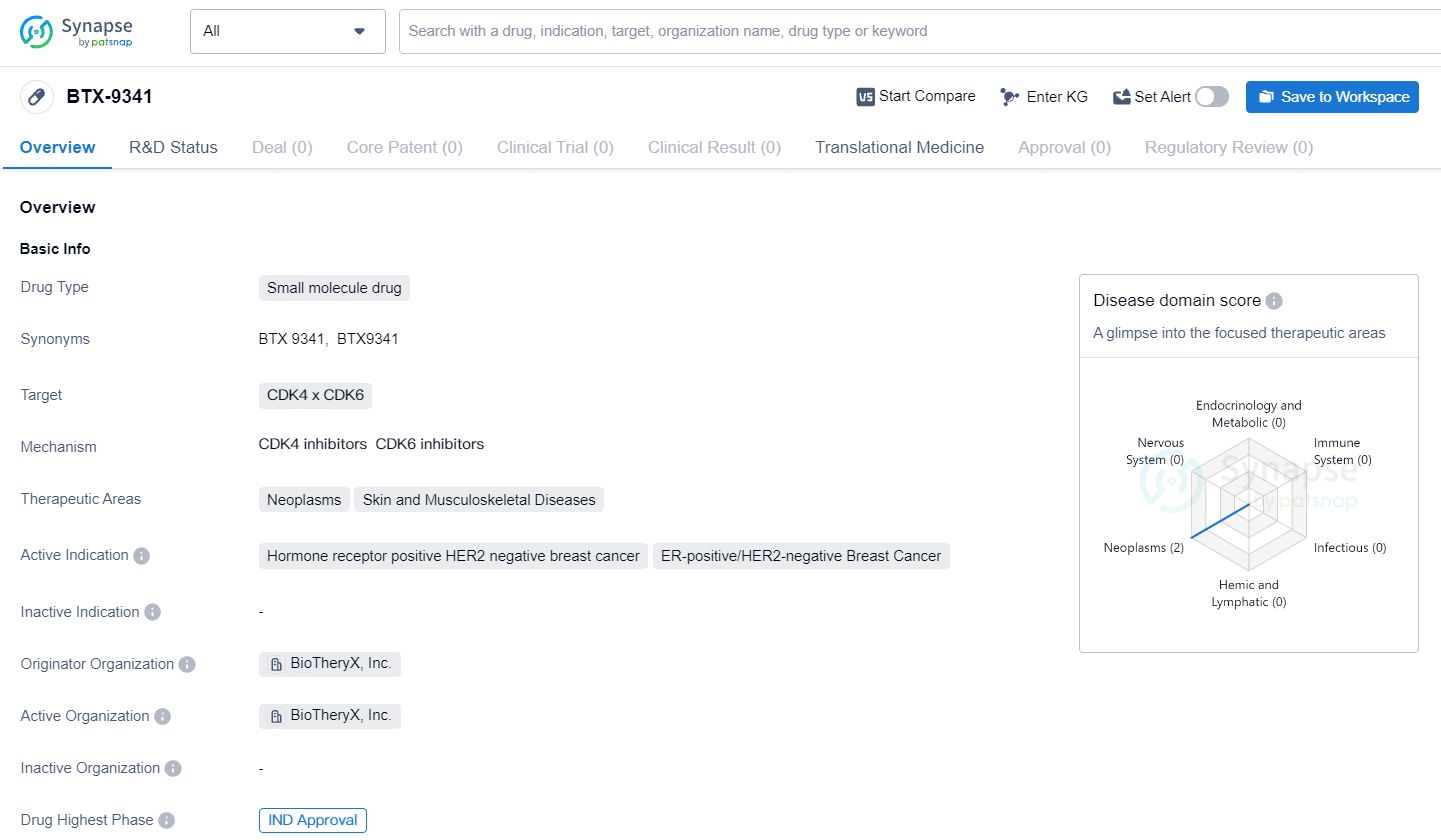
Biotheryx, Inc., a biopharmaceutical firm engaged in the development and discovery of innovative protein degraders with an emphasis on established targets for cancer and inflammatory conditions, revealed that their Investigational New Drug application for BTX-9341, an innovative bifunctional degrader targeting cyclin-dependent kinase 4/6 (CDK4/6), has been approved by the U.S. Food and Drug Administration.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The achievement of FDA approval for the IND application of BTX-9341 signifies a pivotal development for Biotheryx, highlighting our pledge to progress in the field of novel, orally administered targeted protein degraders. "This regulatory milestone propels us from encouraging preclinical findings toward the initiation of clinical trials, positioning us to evaluate BTX-9341's efficacy in delivering real-world benefits to individuals confronted with breast cancer," stated Leah Fung, Ph.D., CEO at Biotheryx.
The upcoming Phase 1 study will commence with a dose escalation phase for BTX-9341 as a standalone treatment and proceed to a dose expansion phase, where BTX-9341 will be combined with fulvestrant. The primary areas of clinical focus will be on assessing tolerability, biological effects, and early signs of effectiveness.
BTX-9341 represents a groundbreaking, orally administered agent that targets and degrades both cyclin-dependent kinase 4 and cyclin-dependent kinase 6. These kinases are critical in various cancers and have been clinically proven effective targets in specific breast cancer cases. In preclinical studies on breast cancer, BTX-9341 has shown enhanced performance compared to traditional CDK4/6 inhibitors by efficiently and selectively degrading CDK4 and CDK6. It also exhibits strong inhibition of Cyclin E and CDK2 transcription, leading to cell cycle arrest, and ultimately demonstrating superior efficacy in in vivo breast cancer models.
Moreover, BTX-9341 stands out because it has shown potential in circumventing major resistance challenges that undermine current CDK4/6 inhibitor treatments. Approximately 20% of patients exhibit inherent resistance, and nearly 70% develop resistance after treatment with CDK4/6 inhibitors. Furthermore, BTX-9341 is characterized by its notably improved ability to cross the blood-brain barrier, potentially increasing its therapeutic reach and effectiveness.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
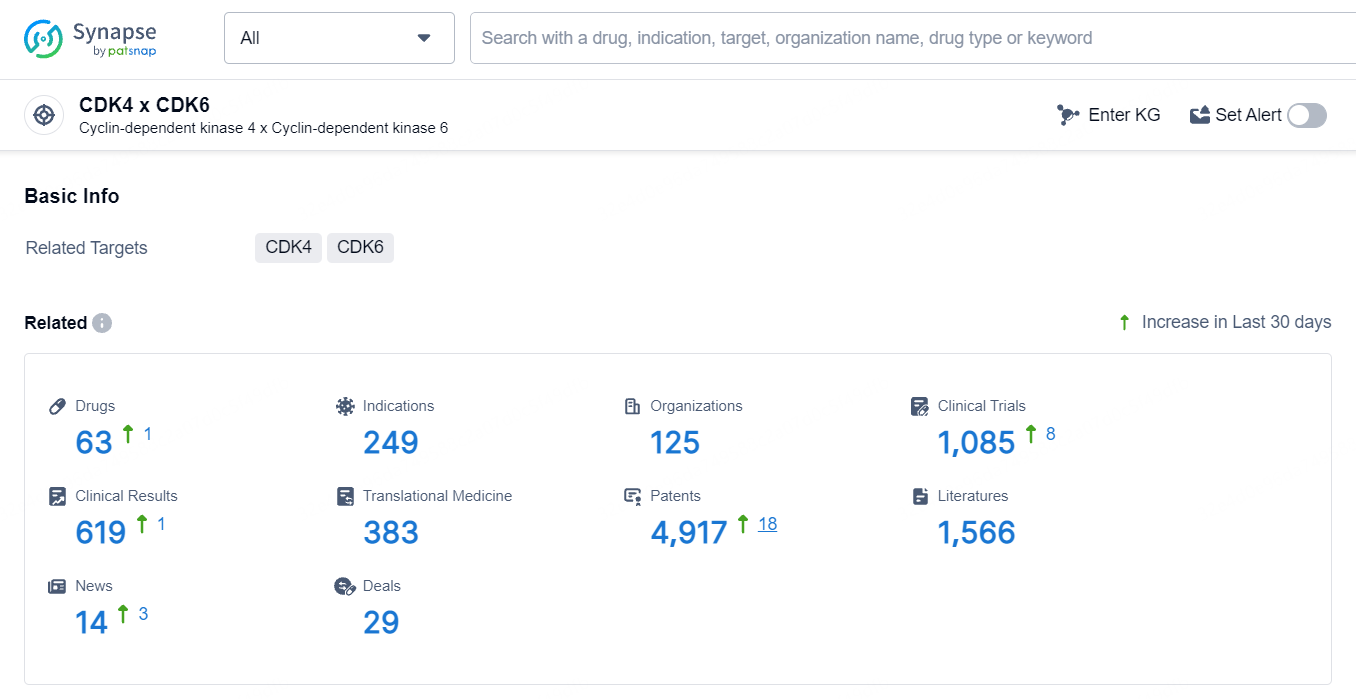
According to the data provided by the Synapse Database, As of May 11, 2024, there are 63 investigational drugs for the CDK4 and CDK6 targets, including 249 indications, 125 R&D institutions involved, with related clinical trials reaching 1084, and as many as 4917 patents.
BTX-9341 targets CDK4 and CDK6 and is being developed for the treatment of neoplasms, skin diseases, and musculoskeletal diseases. The active indications for BTX-9341 include breast cancer, specifically ER-positive/HER2-negative breast cancer, and solid tumors. Currently, the drug is in the preclinical phase, and further research and testing are required before it can progress to human clinical trials.