Boehringer Ingelheim: Continuously Investing in the Development of Schizophrenia Treatment Drugs Targeting GPR52
On March 11th, German pharmaceutical giant Boehringer Ingelheim reached a global strategic collaboration and exclusive option agreement with Japanese biotech company Sosei Group Corporation. The two parties will work together to develop and commercialize the first of its kind innovative drug, a GPR52 agonist, targeting all symptoms of schizophrenia.

Schizophrenia is a severe disorder affecting approximately one in every hundred people worldwide. Its clinical presentation primarily includes three categories of symptoms: positive symptoms (such as hallucinations, delusions, and paranoia), negative symptoms (such as social withdrawal and emotional flatness), and cognitive symptoms (such as poor concentration, reduced planning abilities, and memory decline). Currently, although antipsychotic medications can stabilize positive symptoms, they are associated with significant side effects, and there are no effective approved drugs on the market specifically for negative and cognitive symptoms.
The core of this collaboration is based on the GPR52 agonist series under investigation by Sosei Heptares, which aims at a novel G protein-coupled receptor (GPCR) target. GPR52 is distributed in two key areas of the brain—the striatum, which is responsible for driving positive symptoms, and the prefrontal cortex, which leads to negative and cognitive symptoms. By activating GPR52, this new therapy has the potential to improve the three types of symptoms simultaneously, offering an unprecedented precision treatment approach. The activation of GPR52 may alleviate striatal activity while enhancing the function of the prefrontal cortex, thereby achieving a more precise intervention for the comprehensive symptoms of schizophrenia.

According to the terms of the collaboration agreement, Sosei Heptares has entered into a lucrative financial transaction with Boehringer Ingelheim. Upon signing the agreement, Sosei Heptares will receive an upfront payment of 25 million euros from Boehringer Ingelheim, and should Boehringer Ingelheim decide to exercise its exclusive option right, upon meeting certain conditions, it will be eligible for an option fee of 60 million euros. Furthermore, Sosei Heptares could receive cumulative additional payments of up to 670 million euros for further development, regulatory approvals, and commercialization processes for the GPR52 agonist series, and will collect royalties on Boehringer Ingelheim's product sales based on the conventional tiered-rate of clinical phase assets.
The agreement stipulates that Boehringer Ingelheim has an exclusive option post the completion by Sosei Heptares of its ongoing phase I and subsequent Ib clinical trials of HTL0048149 (the first-in-class GPR52 agonist), as well as the necessary preparations for entering phase II clinical research. Until the expected option exercise by 2025, Sosei Heptares will continue to control and act as the sponsor for these trials. Once the license becomes effective, the licensed product portfolio will include not only HTL0048149, which is already in clinical development, but also a variety of differentiated backup compounds developed by Sosei Heptares using its proprietary StaR® technology and structure-based drug design (SBDD) platform.
About GPR52
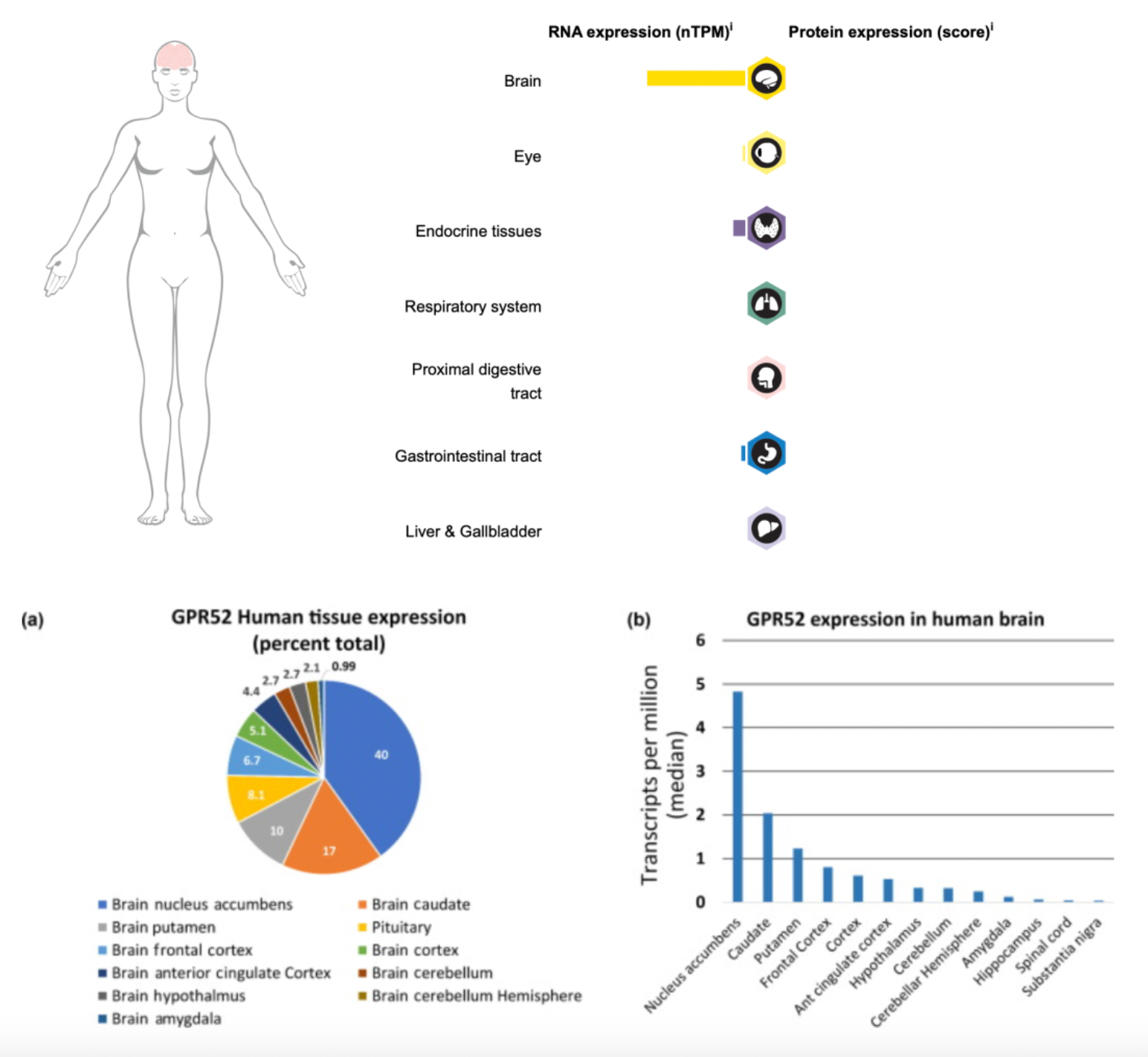
GPR52 is an orphan G protein-coupled receptor (GPCR) that was initially identified by researchers in 1999 through homology searches comparing public high-throughput genomic databases as a novel human GPCR gene. With the advancement of genomic research, Genome-Wide Association Studies (GWAS) have implicated GPR52 as a risk gene for schizophrenia. GPR52 is highly expressed in the brain, particularly in the striatum and the prefrontal cortex regions, accounting for about 70% of the total GPR52 expression in human tissues. This feature makes it a potential new therapeutic target for a range of neurological and psychiatric disorders.
Studies have shown that GPR52 knockout mice, compared to wild-type mice, exhibit reduced expression of D2R mRNA and enkephalin in the striatum region. This suggests that the absence of GPR52 enhances D2R signaling within the basal ganglia, thereby lowering the activity level of striatopallidal neurons. Histological research using genetically modified mouse models has demonstrated that transgenic mice overexpressing GPR52 exhibit effects similar to antipsychotic drugs, while GPR52 knockout mice present phenotypes associated with psychiatric illness. In vitro experiments have shown that using alternative GPR52 agonists can increase cAMP levels and demonstrate antipsychotic-like actions, capable of suppressing hyperactive behavior induced by amphetamine or methamphetamine. There may be crosstalk between GPR52 and D2R mediated through a cAMP-dependent signaling pathway; therefore, GPR52 agonists functionally resemble D2R antagonists.
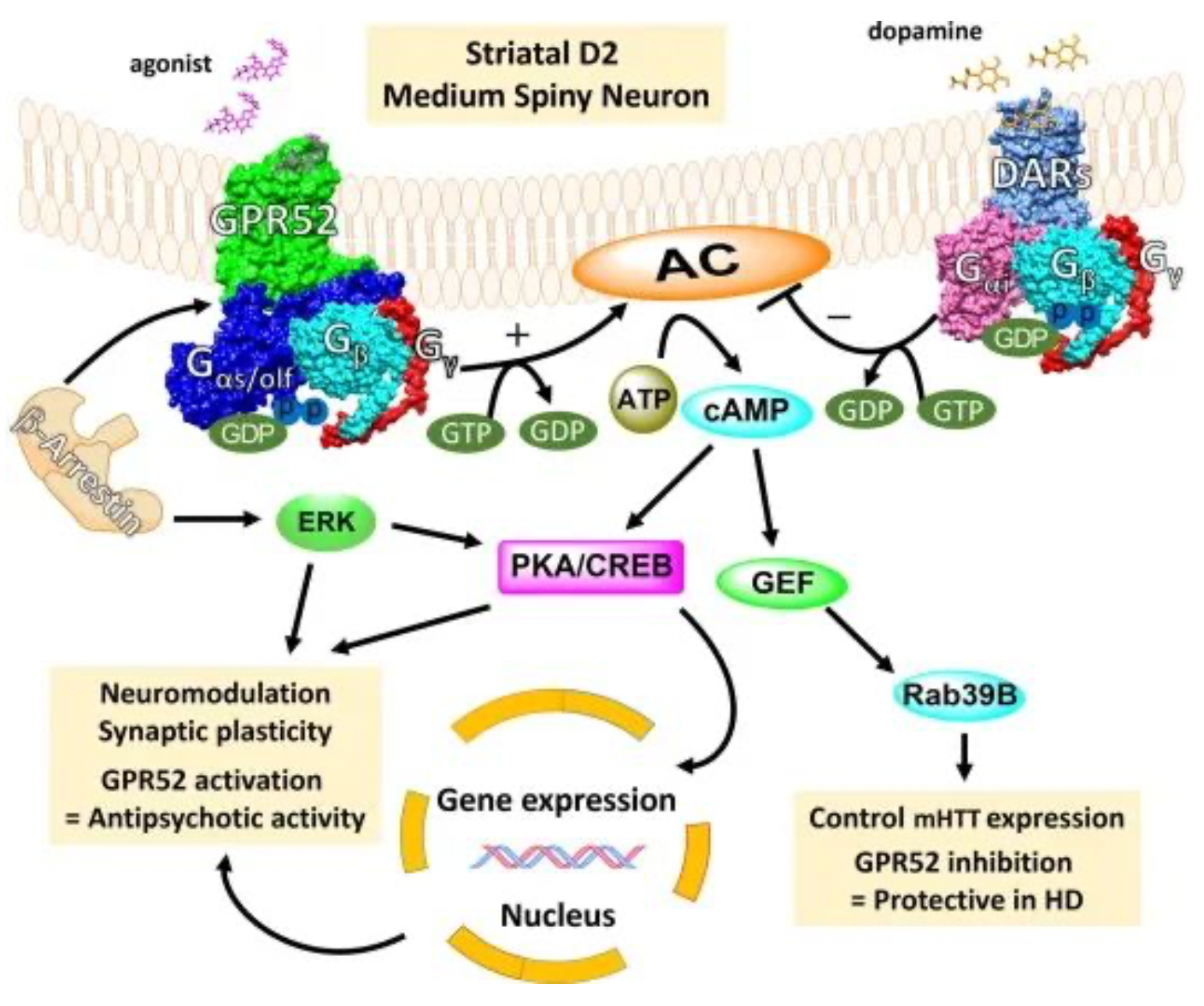
GPR52 is progressively emerging as a novel and promising therapeutic target for the treatment of mental illnesses, including schizophrenia and substance abuse disorders.
Regarding New Drug Discovery for GPR52
According to the synapse database statistics, there are several new drug development projects globally targeting GPR52, all focusing on the development of small molecule agonists for GPR52. Potential clinical indications include schizophrenia, mild cognitive impairment, disruptive, impulse control, and conduct disorders, among others.
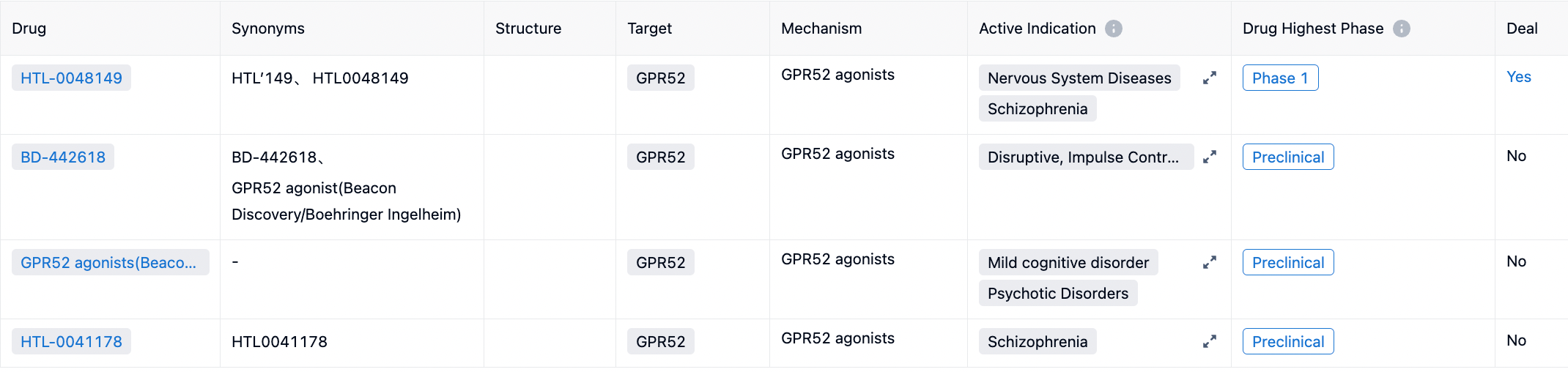
HTL0048149 (HTL'149) is a selective GPR52 agonist developed by Sosei Heptares using its proprietary StaR® technology platform for structure-based drug design. As of 2023, HTL0048149 has entered the first-in-human clinical trial phase.
It is reported that HTL'149, as an oral medication administered once daily, specifically targets GPR52 and is characterized by its antipsychotic effects and cognitive function improvement. HTL'149 aims to treat the positive symptoms associated with schizophrenia (such as psychosis, delusions, hallucinations), negative symptoms (such as social withdrawal and emotional blunting), as well as cognitive impairments (such as lack of concentration, diminished planning abilities, and memory loss). In addition, the drug seeks to reduce the adverse side effects caused by some antipsychotic medications currently on the market. Through this novel mechanism of action, HTL'149 is expected to meet the needs of a large number of schizophrenia patients who have not responded well to existing treatments or are unable to continue using antipsychotic medications due to tolerance issues, as existing antipsychotic drugs typically cannot effectively improve patients' negative and cognitive symptoms.
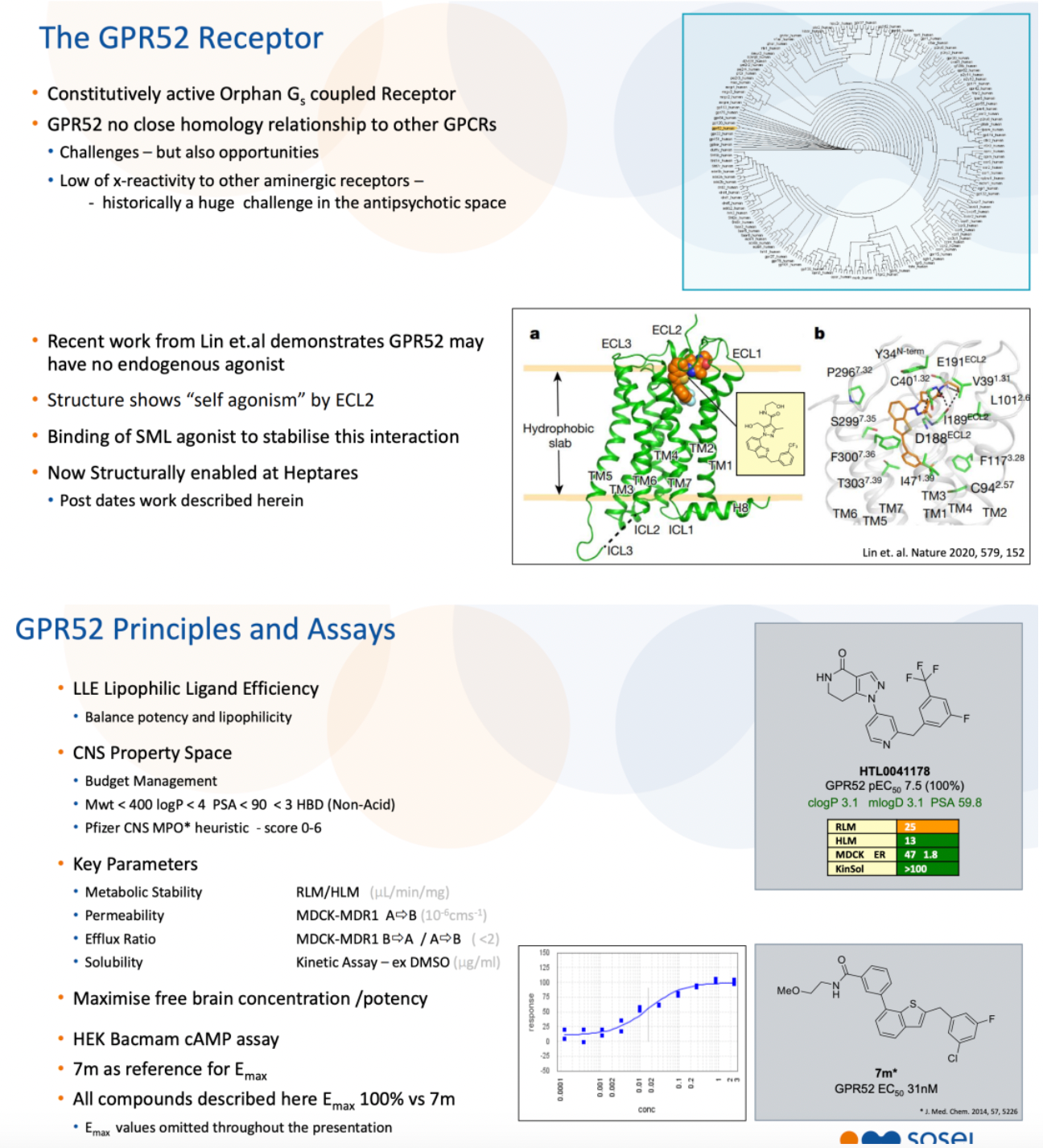
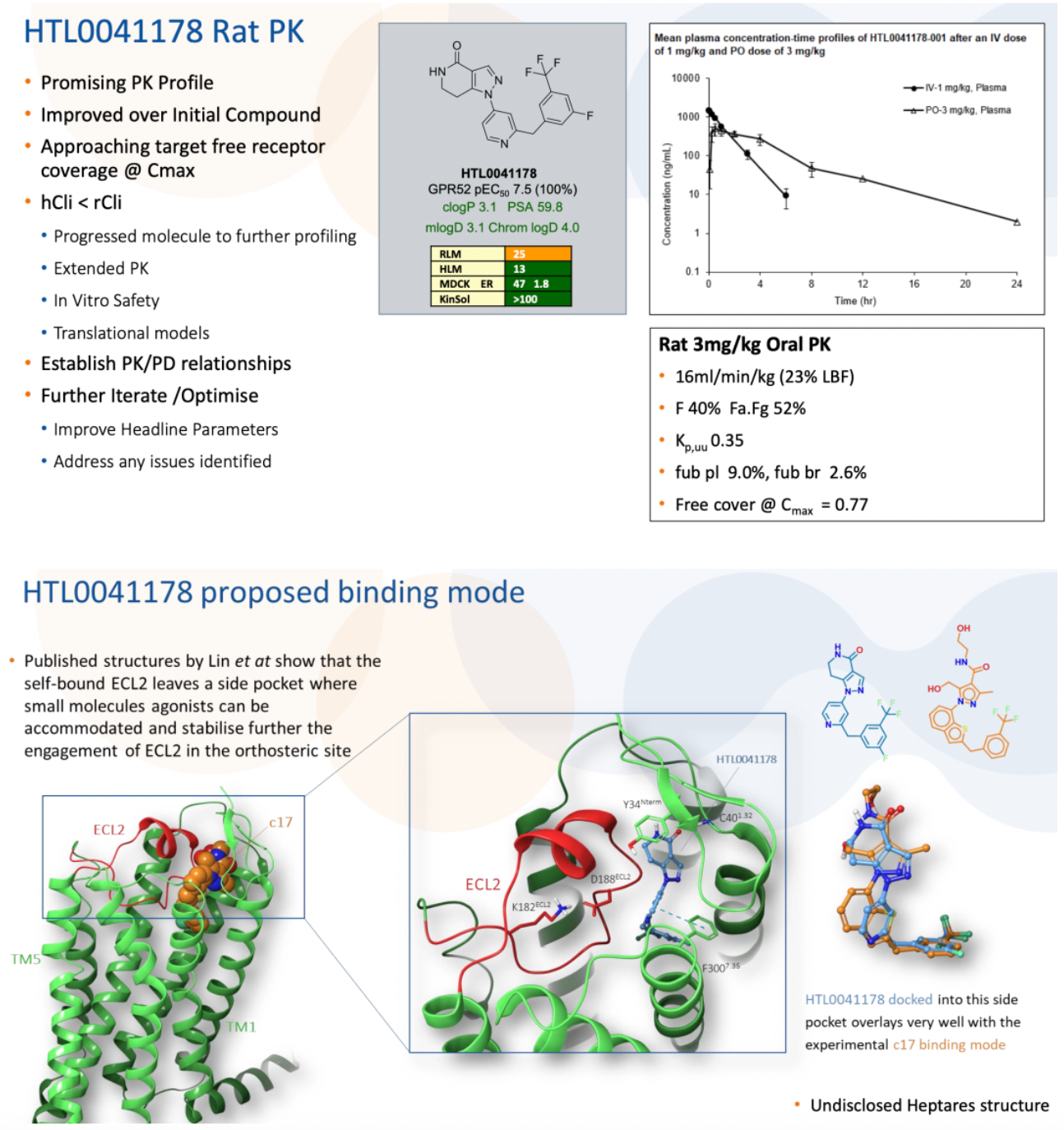
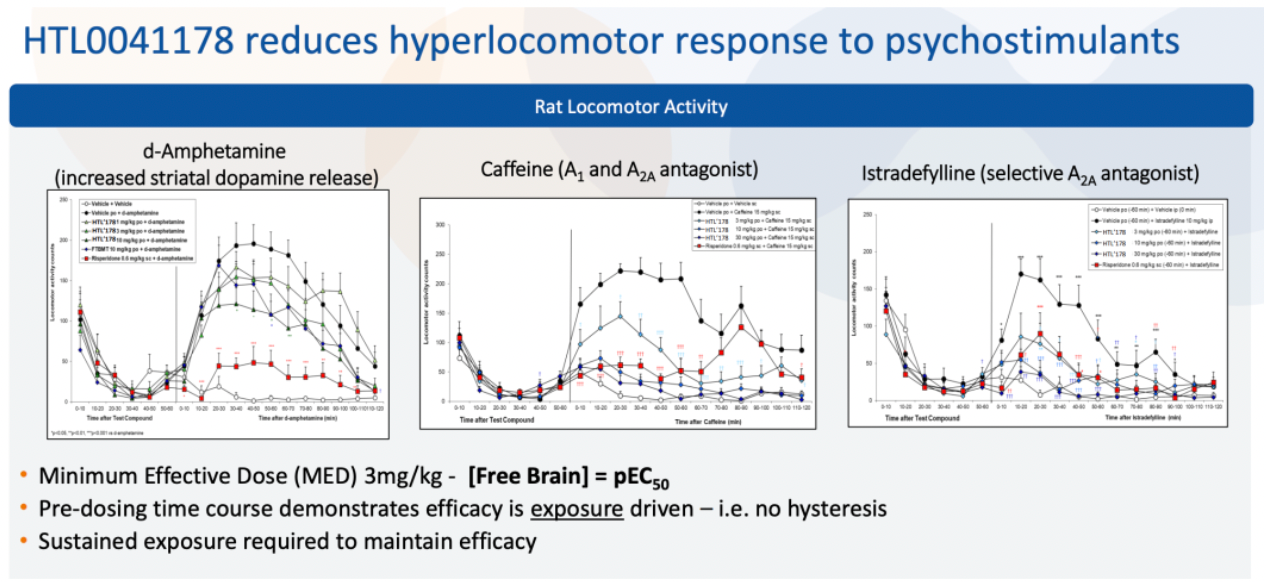
HTL0041178 is another highly effective GPR52 receptor agonist developed by Sosei Heptares. It has a favorable pharmacokinetic profile and has demonstrated oral activity in preclinical models. This compound was selected through a carefully designed optimization strategy based on molecular properties, aiming to balance potency, metabolic stability, solubility, permeability, and P-glycoprotein efflux effects.
In March 2023, Sosei Heptares collaborated with Syngene to publish an article in the journal "ACS Med. Chem. Lett." introducing the development of HTL0041178.
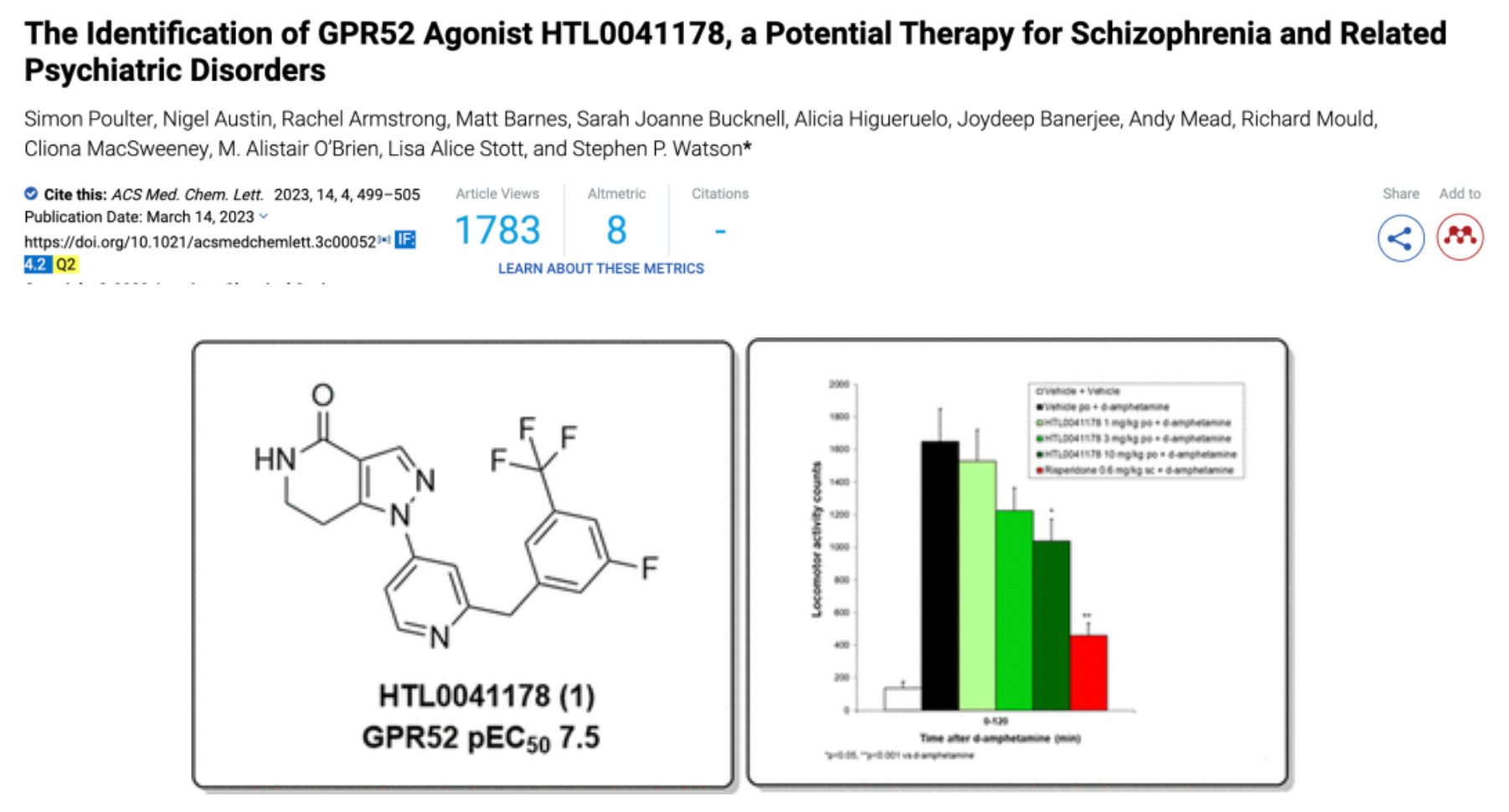
According to another document released by Sosei Heptares in 2021, the development of the GPR52 agonist HTL0041178 is introduced.
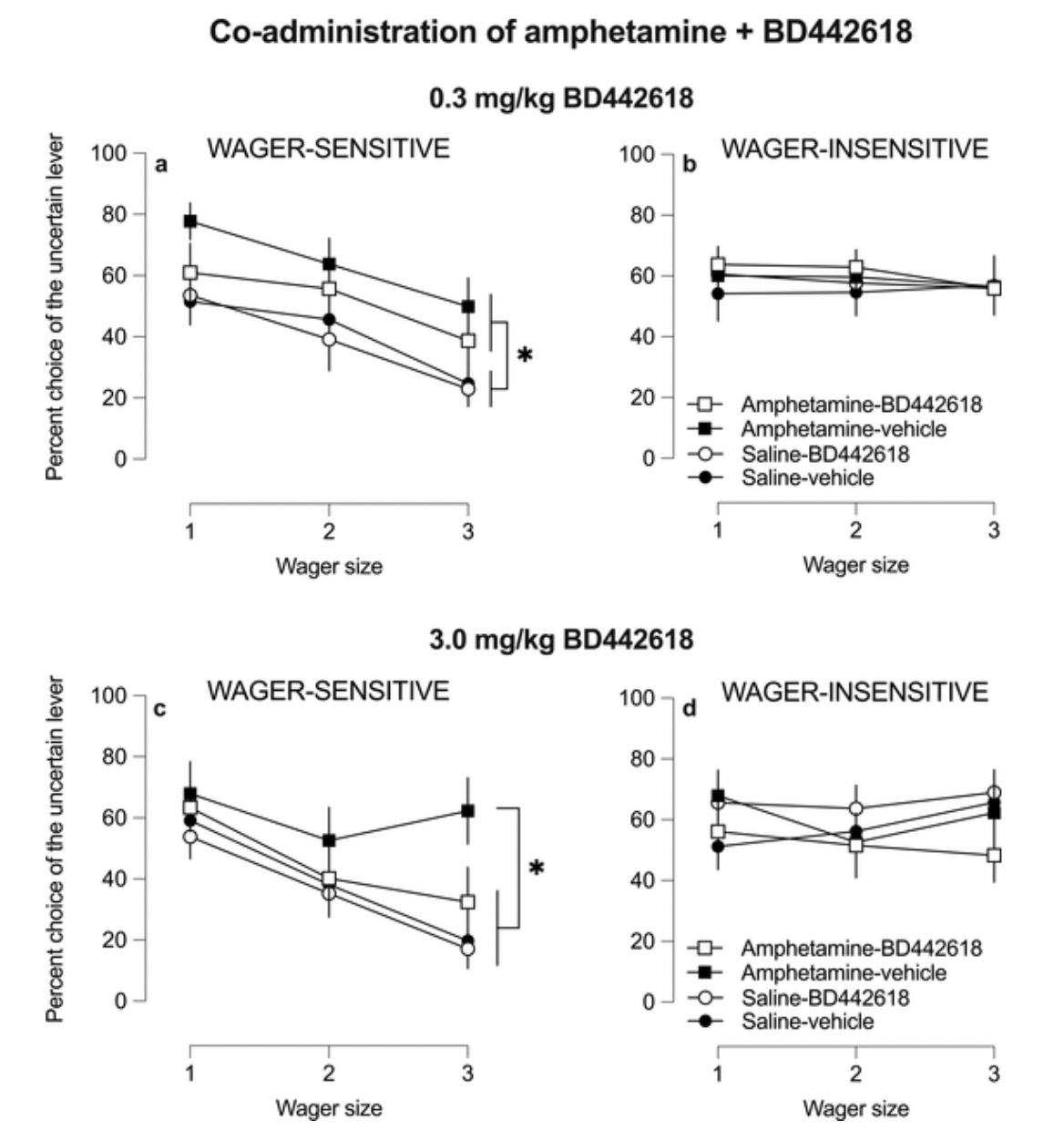
BD-442618 is a GPR52 agonist developed jointly by Beacon Discovery and Boehringer Ingelheim. According to a study published in 2021, researchers investigated whether two potent small molecule GPR52 agonists (BD442618 and BD502657) could inhibit the enhanced preference for uncertain outcomes caused by acute amphetamine (a central nervous system stimulant frequently used as an excitant or for treating Attention Deficit Hyperactivity Disorder) and chronic administration of ropinirole (a dopamine receptor agonist primarily used for treating Parkinson's Disease and Restless Legs Syndrome), without affecting the baseline decision-making pattern.
The results demonstrated that BD442618 did not alter baseline decision-making, yet it successfully attenuated the increased preference for uncertainty induced by acute amphetamine and chronic ropinirole administration. Similarly, BD502657 also eliminated the effects associated with chronic ropinirole exposure.
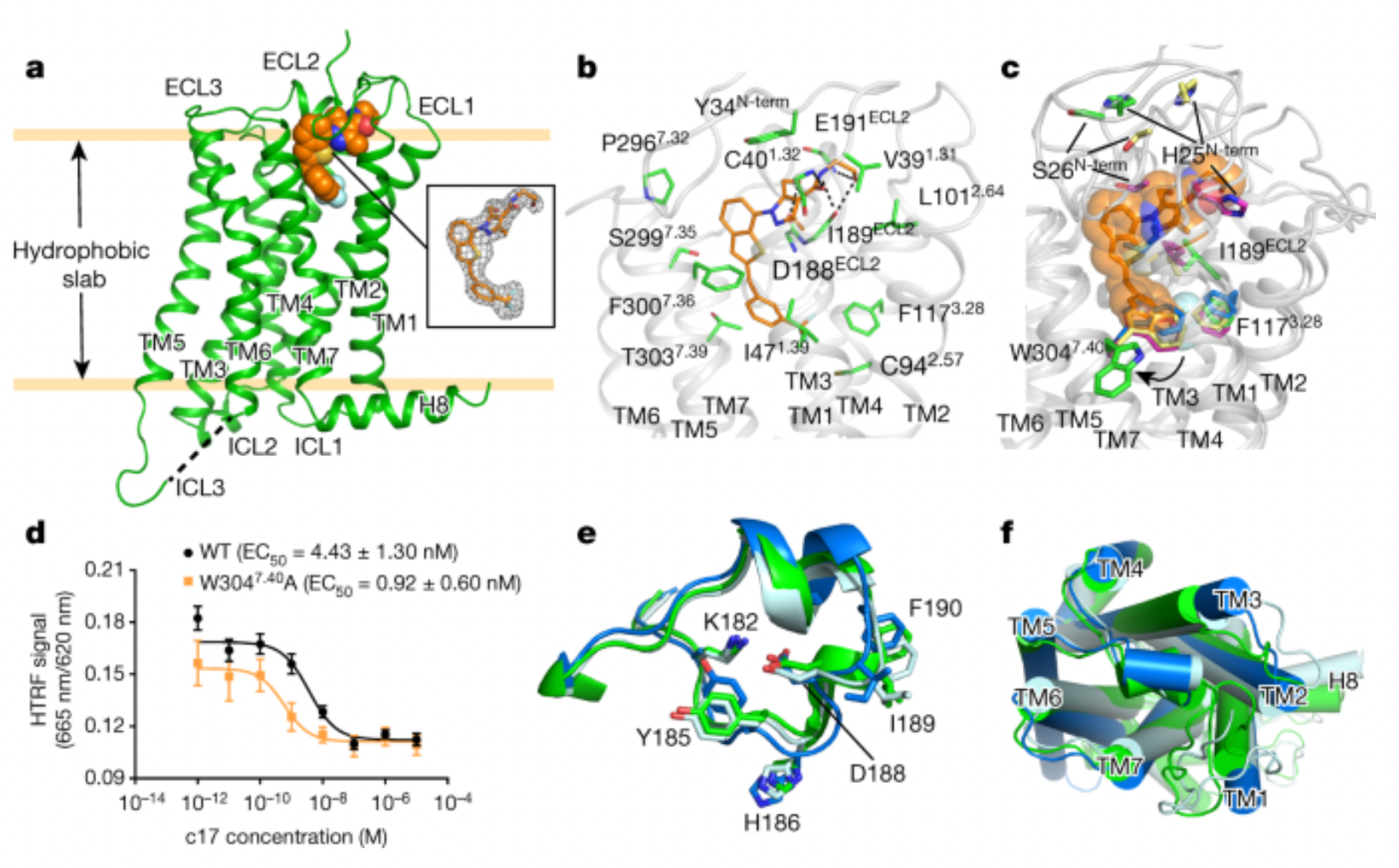
Additionally, C17 and compound 1b are two GPR52 agonists developed by Takeda Pharmaceutical Company. Researchers initially reported the first orally active GPR52 agonist, compound 1b, but its high lipophilicity and poor water solubility continue to pose challenges during the screening of candidate drugs. Based on compound 1b, researchers designed and synthesized a novel series of 1-(benzothiophene-7-yl)-1H-pyrazole as GPR52 agonists. Within this series, compound C17 demonstrated an EC50 value of 21 nM and an Emax value of 103% for GPR52, with a logD value of 2.21 and a solubility of 21 μg/mL at pH 6.8. This compound C17 was able to dose-dependently inhibit methamphetamine-induced hyperactivity in mice.

In 2020, researchers reported the first high-resolution complex structure of a GPR52 agonist, C17, bound to GPR52 (DOI: 10.1038/s41586-020-2019-0).