Initial Positive Clinical Trial Results for CS-1103: A Novel Treatment for Meth and Fentanyl Overdose
Clear Scientific, Inc., a biopharma company in the clinical stage concentrating on treatments for ailments and diseases related to an overabundance of toxic substances in the body, revealed that the topline results from the First-in-Human Phase 1 clinical trial of their therapy CS-1103 indicate a positive safety and tolerability profile.
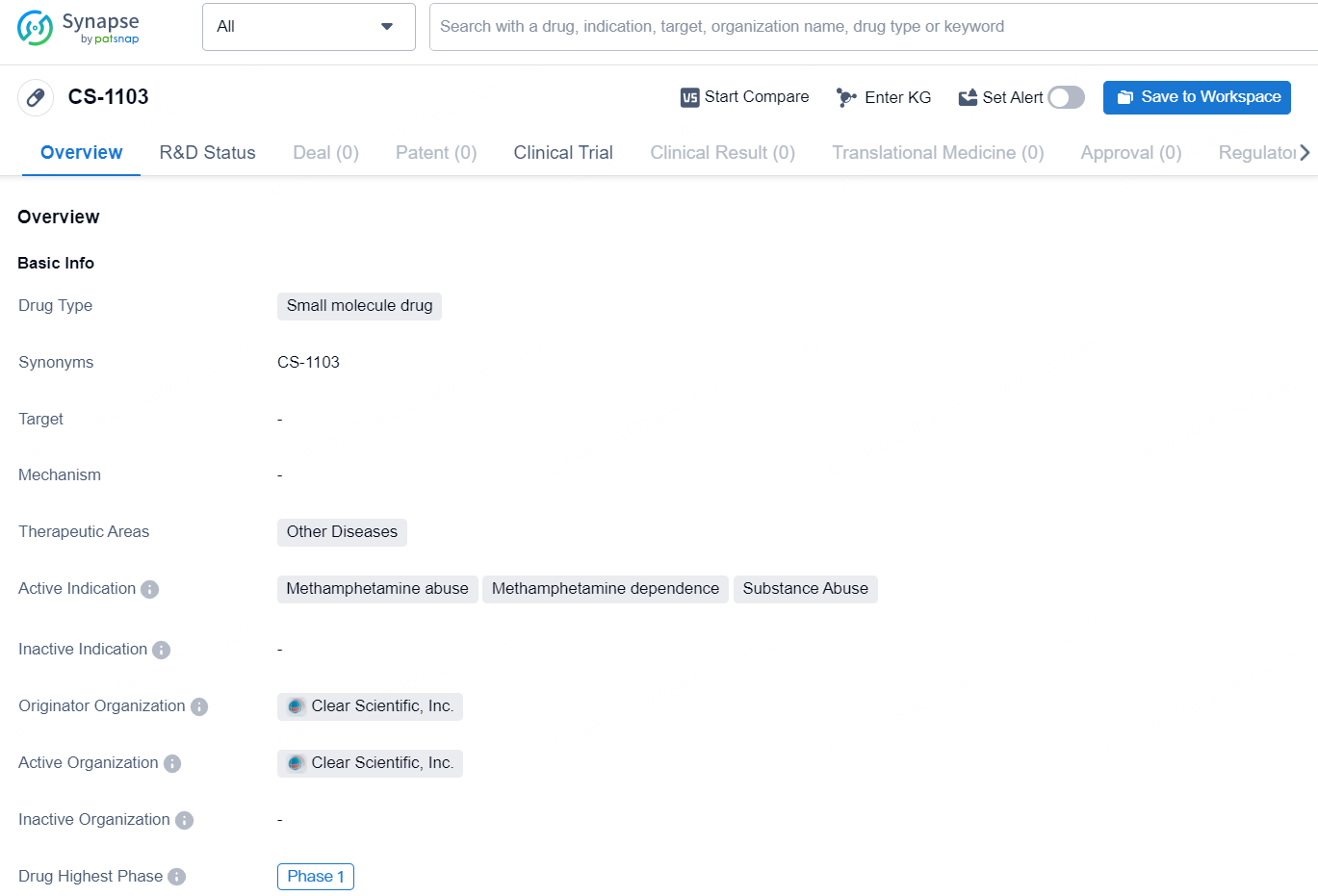
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
All thirty-two healthy volunteers who were recruited and divided into four groups successfully completed the Phase 1 randomized, double-blind, placebo-controlled study focusing on single ascending doses to assess safety, tolerability, and pharmacokinetics. In this investigation, intravenous administration of single doses of CS-1103 was found to be well tolerated with no serious adverse events observed.
The findings from the Phase 1 study validate the dosing protocol for the upcoming Phase 2 studies addressing methamphetamine and fentanyl overdose, which are scheduled for early 2025.
“These Phase 1 results mark a significant milestone. When combined with our pre-clinical data, they convincingly demonstrate the safety and efficacy of CS-1103. Given these favorable outcomes, we are moving towards an accelerated registration pathway with plans to commence Phase 2 studies in early 2025. We extend our gratitude to the National Institute on Drug Abuse (NIDA) of the NIH for their persistent support,” stated Shekar Shetty, CEO of Clear Scientific.
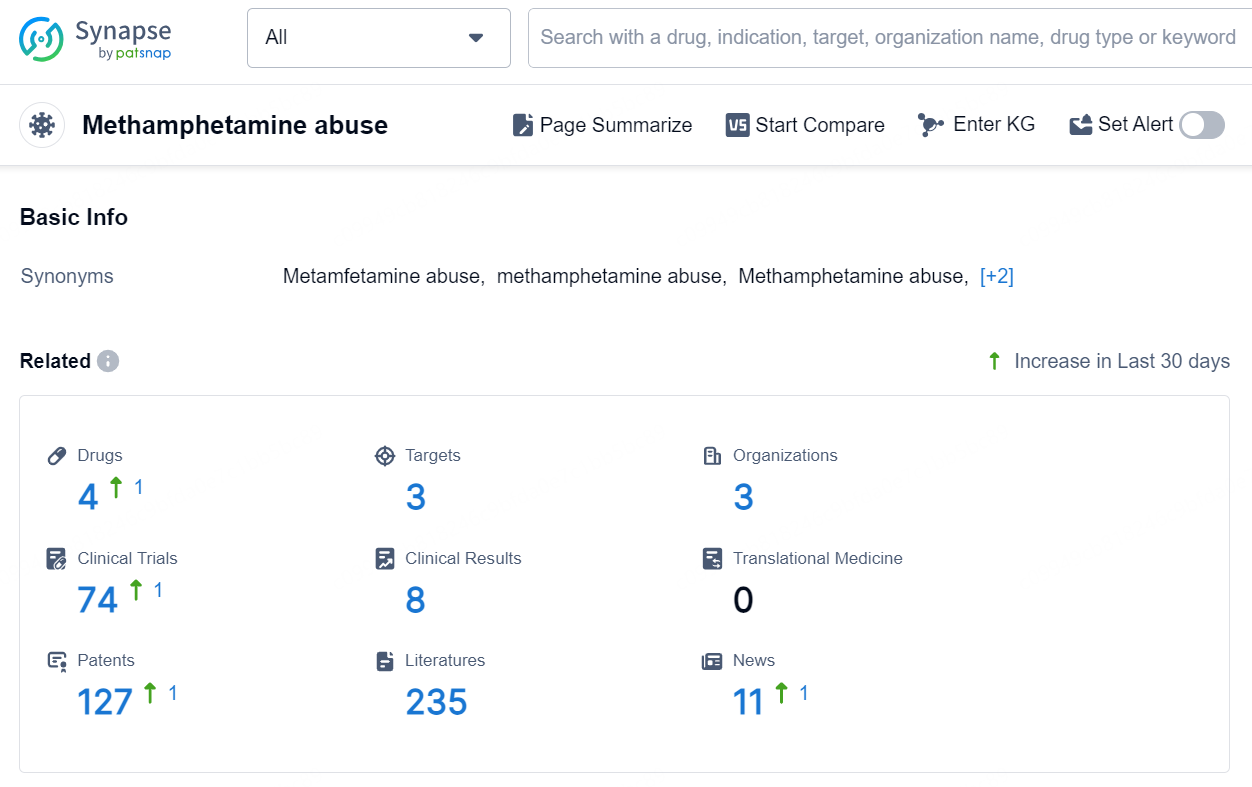
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 12, 2024, there are 4 investigational drugs for the Methamphetamine abuse, including 3 targets, 3 R&D institutions involved, with related clinical trials reaching 74, and as many as 127 patents.
CS-1103 is a small molecule drug developed by Clear Scientific, Inc. The drug falls under the therapeutic area of Other Diseases, with active indications including Methamphetamine abuse, Methamphetamine dependence, and Substance Abuse. As of now, the highest phase of development for CS-1103 is Phase 1.