Phanes Therapeutics Reveals Initial Patient Treated in PT886 and Chemotherapy Clinical Trial
Phanes Therapeutics, Inc. (Phanes), a biotech company specializing in advanced drug discovery and development for oncology, reported that the first patient has received a dose in the clinical trial of PT886 alongside chemotherapy. Dosing has been finalized in two separate cohorts: one for initial treatment of pancreatic cancer and the other for second-line treatment of gastric and gastroesophageal junction cancers.
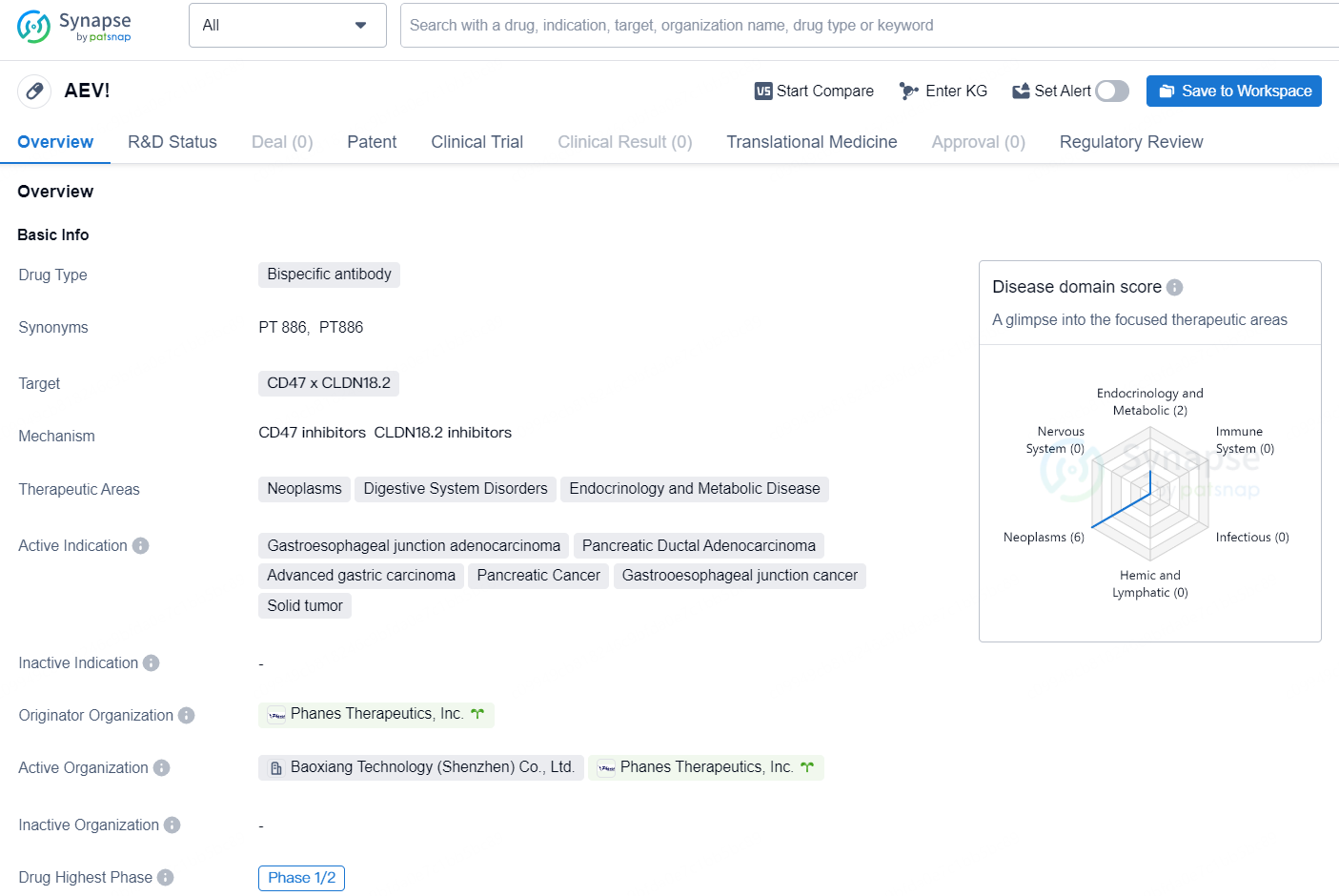
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
PT886 is a pioneering bispecific antibody (bsAb) that resembles native IgG, targeting both claudin 18.2 and CD47. The FDA granted it orphan drug designation (ODD) for pancreatic cancer treatment in 2022 and later gave it Fast Track designation for treating patients with metastatic claudin 18.2-positive pancreatic adenocarcinoma earlier this year. In 2023, Phanes initiated a clinical collaboration with Merck (known as MSD outside of the US and Canada) to investigate PT886 in combination with Merck’s anti-PD-1 therapy, KEYTRUDA® (pembrolizumab).
The ongoing, multi-center Phase I/II clinical trial of PT886, named the TWINPEAK study (NCT05482893), is currently assessing the safety, tolerability, pharmacokinetics, pharmacodynamics, and preliminary efficacy of PT886 in patients with locally advanced or metastatic gastric, gastroesophageal junction, and pancreatic cancers that have either not responded to, are intolerant of, or are deemed inappropriate for all available standard therapies. Additionally, a Phase I clinical study of PT886 is being conducted in China (CTR20241655).
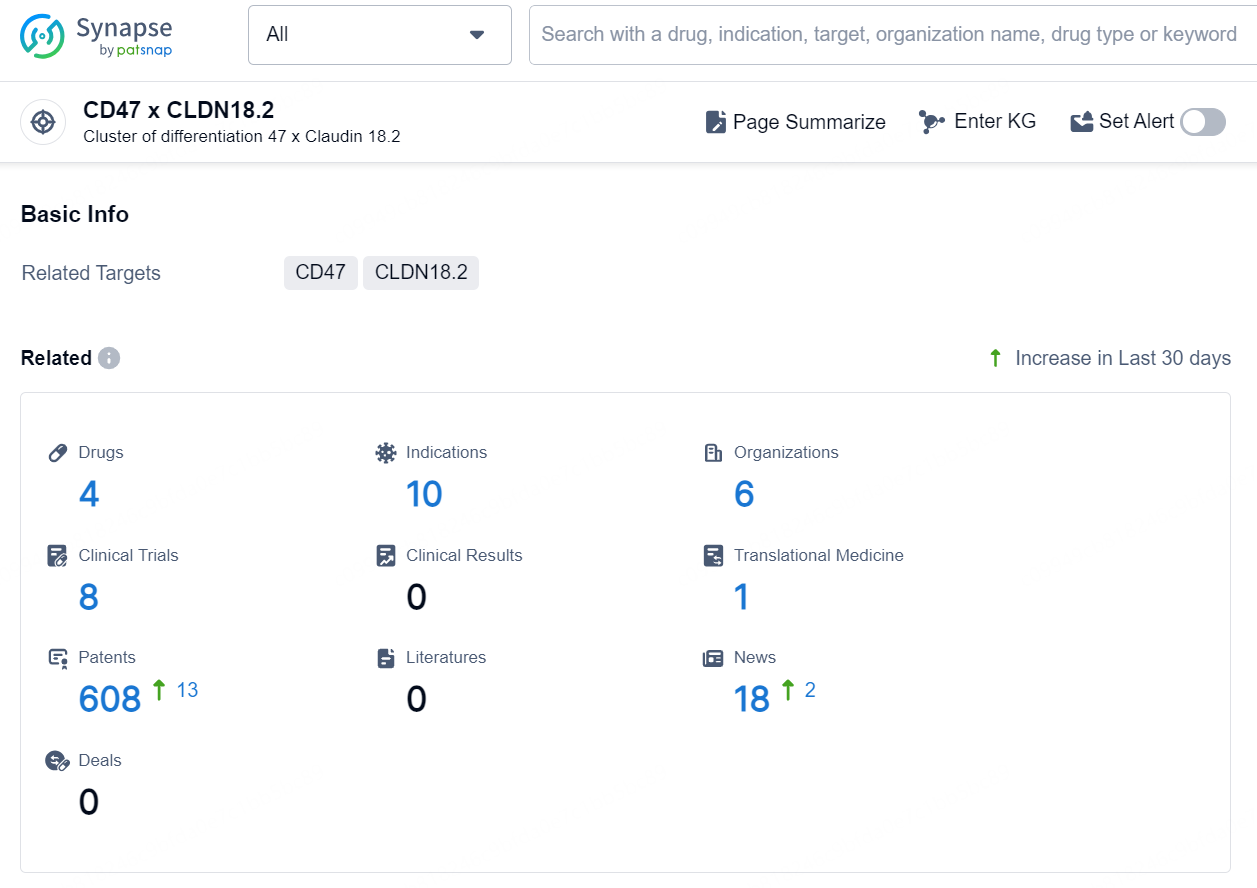
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 12, 2024, there are 4 investigational drugs for the CD47 x CLDN18.2 targets, including 10 indications, 6 R&D institutions involved, with related clinical trials reaching 8, and as many as 608 patents.
PT-886 is a bispecific antibody drug developed by Phanes Therapeutics, Inc. The drug targets CD47 x CLDN18.2 and is being developed for the treatment of several therapeutic areas, including neoplasms, digestive system disorders, and endocrinology and metabolic disease. The active indications for PT-886 include gastroesophageal junction adenocarcinoma, pancreatic ductal adenocarcinoma, advanced gastric carcinoma, pancreatic cancer, gastrooesophageal junction cancer, and solid tumors.