Boosting anti-PD-1 PD-L1 therapy by targeting YTHDF1
Hepatocellular carcinoma (HCC) is the sixth most commonly diagnosed cancer and the third leading cause of cancer-related deaths worldwide. The development of HCC is influenced by both viral and non-viral factors. Non-alcoholic steatohepatitis (NASH) has emerged as a growing risk factor for HCC, as the prevalence of diabetes and metabolic syndrome increases. NASH is a progressive liver disease characterized by the accumulation of excessive triglycerides and is not caused by alcohol consumption. Although NASH is linked to the development of HCC, the molecular mechanisms underlying these diseases are not fully understood, which has hindered the development of effective treatments.
Handicapping anti-PD1 therapy
Currently, there are no approved drugs or biological therapies specifically targeting NASH-related HCC (NASH-HCC). This deadly disease urgently requires new treatment methods. Immune checkpoint blockade (ICB) using monoclonal antibodies that target programmed cell death protein-1 (PD-1), programmed cell death-ligand 1 (PD-L1), or cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) has recently become a treatment option for HCC.
However, NASH-HCC's poor response to ICB treatment is due to NASH-related immune changes in the liver. Therefore, identifying potential therapeutic targets is critical to overcoming resistance to ICB treatment in NASH-HCC.
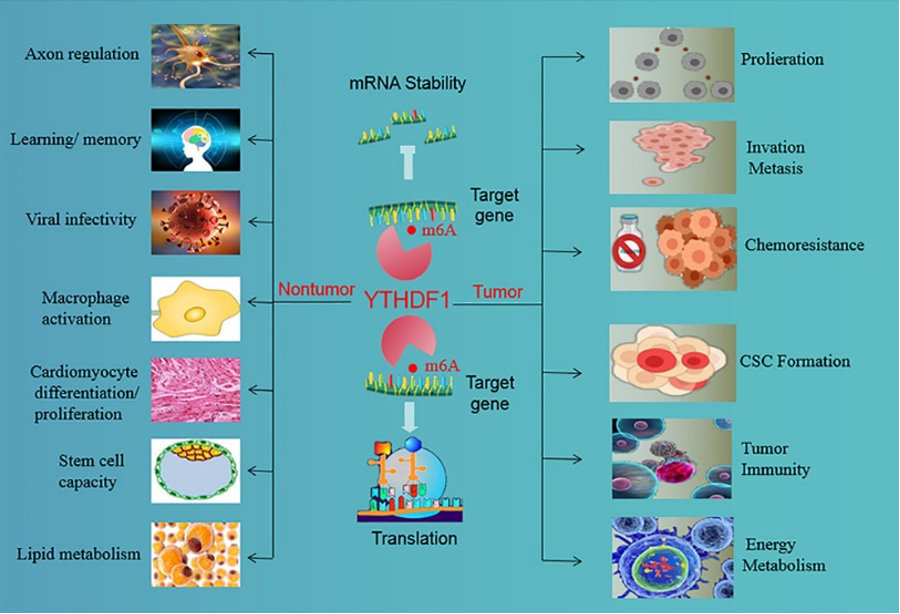
N6-methyladenosine (m6A) is the most prevalent chemical modification in eukaryotic mRNA and is known to play a crucial role in post-transcriptional regulation. m6A modification regulates RNA fate by controlling RNA stability, processing, transport, localization, translation, and RNA-protein interactions. YTH domain-containing family protein 1 (YTHDF1) is a translation-promoting m6A reader that targets mRNAs in the cytoplasm and contributes to tumor progression by regulating cell proliferation, DNA damage, and immunity.
YTHDF1 deficiency in mice enhances antigen-specific CD8+ T cell-mediated anti-tumor responses in colorectal and gastric cancers, making it a potential therapeutic target for cancer immunotherapy. However, the role of YTHDF1 in the pathogenesis of HCC, particularly NASH-HCC, and its regulation of the tumor immune microenvironment are currently not well understood.
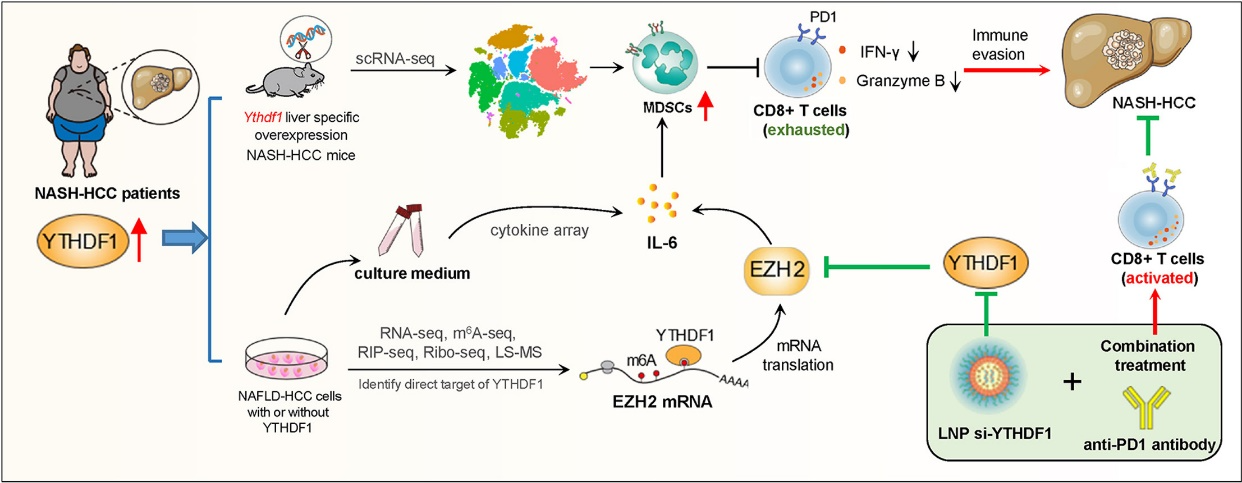
A recent study published in Journal of Hepatology provides valuable insights into YTHDF1’s role in the progression of NASH-HCC. It is the first to demonstrate that tumor-intrinsic YTHDF1 is linked to the accumulation of myeloid-derived suppressor cells (MDSCs) in the NASH-HCC microenvironment. In transgenic mice with liver-specific YTHDF1 overexpression, YTHDF1 induces MDSC infiltration and accelerates spontaneous NASH-HCC.
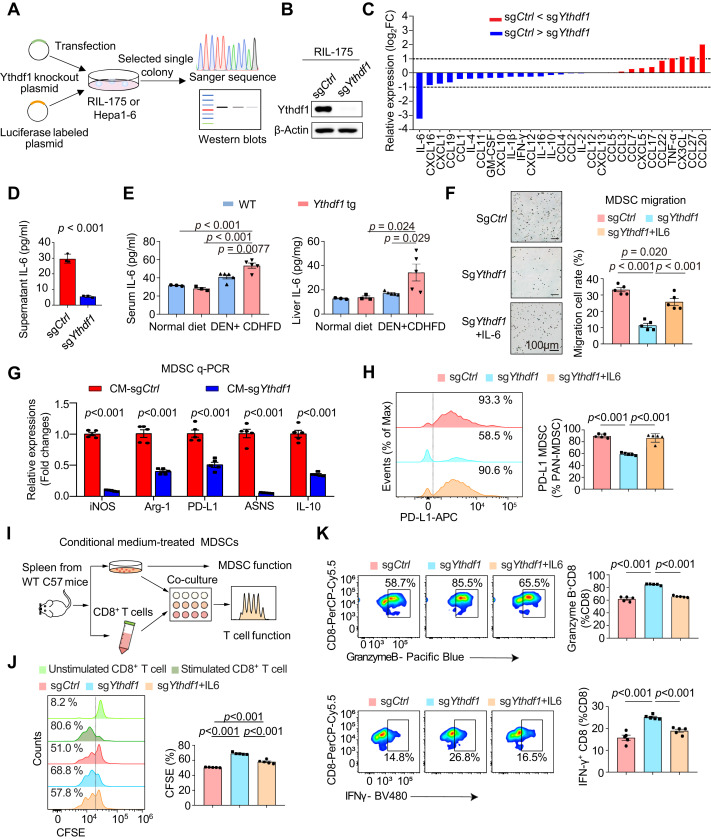
The signaling cascade of YTHDF1 activates the EZH2-IL-6 signaling pathway to recruit MDSCs, resulting in T cell dysfunction and promoting tumor growth. Conversely, targeting YTHDF1 with lipid nanoparticle (LNP)-siRNA can significantly enhance the anti-PD-1 blockade effect in NASH-HCC mouse models, suggesting that YTHDF1 is a new target for enhancing the efficacy of immune checkpoint blockade therapy in NASH-HCC.
Targeting the accomplice: YTHDF1
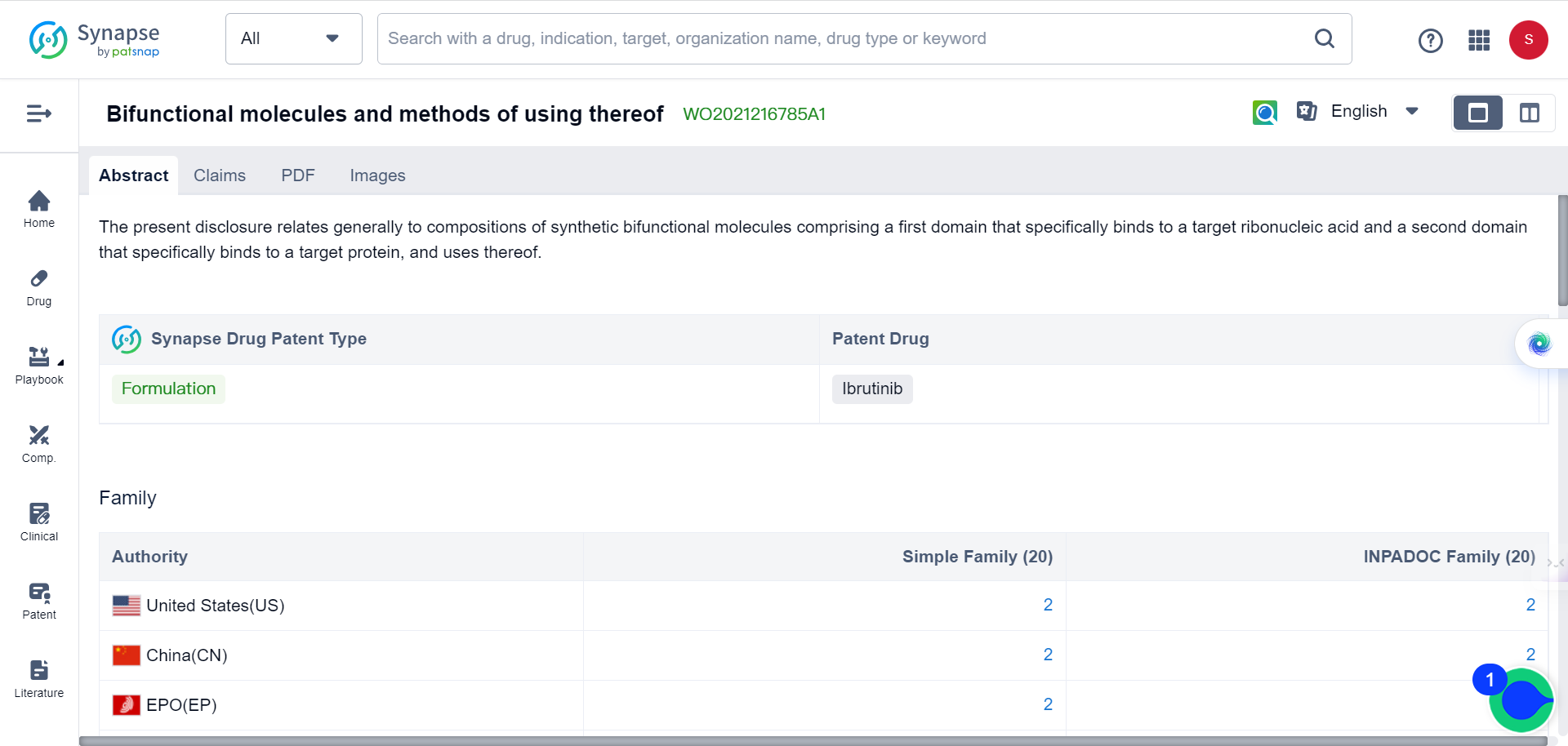
According to the Synapse database, there are currently four patent applications targeting YTHDF1 for cancer therapy. Three of these applications are for the novel use of already existing drugs, while one is for a new formula:
This is not the first time YTHDF1's role in cancer pathology has been elucidated. Last year, a study reported that YTHDF1 promotes breast cancer cell growth in both in vitro and in vivo environments and plays a crucial role in DNA damage repair and chemoresistance. And it seems that YTHDF1 modulates E2F8 mRNA stability and DNA damage repair in a METTL14-dependent manner.
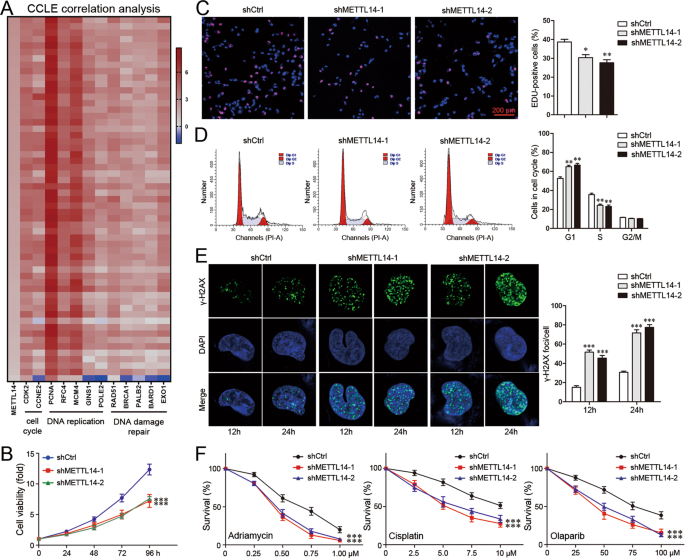
On the other hand, YTHDF1 knockdown has been shown to sensitize breast cancer cells to Adriamycin and Cisplatin, as well as Olaparib, a PARP inhibitor. During the experiments, researchers observed that the knockdown of YTHDF1 in MDA-MB-231 cells resulted in a reduction of S-phase fraction and an increased G1-phase fraction. The YTHDF1 knockdown also downregulated CDK2 and Cyclin E2, while upregulating P21, a CDK inhibitor involved in S-phase.
YTHDF1’s roles in other types of cancer have been revealed as well. A study published earlier this year has suggested that YTHDF1 may play an important role in shaping the immune microenvironment of colorectal cancer (CRC).
In their study, the team of researchers revealed that removing YTHDF1 from mouse CRC cells (both microsatellite stable (MSS) and microsatellite instable-high (MSI-H) varieties) results in an accumulation of myeloid-derived suppressor cells (MDSCs). This accumulation subsequently inhibits the infiltration of T cells in both mouse CRC models and humanized mouse models, demonstrating a deep interconnectedness.
The investigation led to the identification of p65/Rela as a target of YTHDF1. The researchers observed that YTHDF1 encouraged the production of p65 protein, which in turn escalated the regulation of CXCL1. This process amplified the migration of MDSCs through the CXCL1-CXCR2 pathway, resulting in an increased number of MDSCs. These MDSCs then countered the function of CD8+ T cells within the tumor immune microenvironment (TIME).
This intricate research, along with the others discussed here, suggest that the strategic targeting of YTHDF1, potentially using techniques such as CRISPR or VNPs-siYTHDF1, could enhance the efficacy of anti-PD1 therapy in a variety of cancers.

References:
1.Wang L. et al., Targeting N6-methyladenosine reader YTHDF1 with siRNA boosts anti-tumor immunity in NASH-HCC by inhibiting EZH2-IL-6 axis 2, Journal of Hepatology, Available online 17 July 2023.
2.Sun, Y., Dong, D., Xia, Y. et al. YTHDF1 promotes breast cancer cell growth, DNA damage repair and chemoresistance. Cell Death Dis 13, 230 (2022).
3.Bao Y, Zhai J, Chen H, et al, Targeting m6A reader YTHDF1 augments antitumour immunity and boosts anti-PD-1 efficacy in colorectal cancer, Gut 2023;72:1497-1509.