CDK2 Inhibitor——Important Regulator of Cell Cycle Control
Cyclin-dependent kinases (CDKs), a serine/threonine family kinase, play a key role in cell cycle regulation and transcriptional activity. The activity of CDKs depends on their associated Cyclin subunits and non-catalytic regulatory proteins, which form CDK-Cyclin heterodimer complexes, playing a crucial role in regulating different biological processes. These processes include angiogenesis, hematopoiesis, gene transcription, apoptosis, neuronal activity, metabolism, DNA repair, exocytosis, and spermatogenesis.
To date, at least nine types of CDKs have been identified in humans, labeled as CDK1-CDK9. In different tumor cells, cell cycle dysregulation often accompanies changes in CDK activity and/or its interacting proteins. Hence, the development of novel CDK inhibitors becomes an effective approach to combat various malignancies.
CDK2, or cyclin-dependent kinase 2, is a crucial protein involved in cell cycle regulation in the human body. It plays a pivotal role in controlling the progression of cells from the G1 phase to the S phase, allowing DNA replication and cell division to occur. CDK2 forms complexes with cyclins, which activate its kinase activity, leading to the phosphorylation of various target proteins involved in cell cycle progression. Dysregulation of CDK2 activity has been implicated in numerous diseases, including cancer, making it an attractive target for therapeutic interventions aimed at controlling cell proliferation and promoting healthy cell cycle progression.
CDK2 Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 10 Sep 2023, there are a total of 64 CDK2 drugs worldwide, from 72 organizations, covering 69 indications, and conducting 75 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.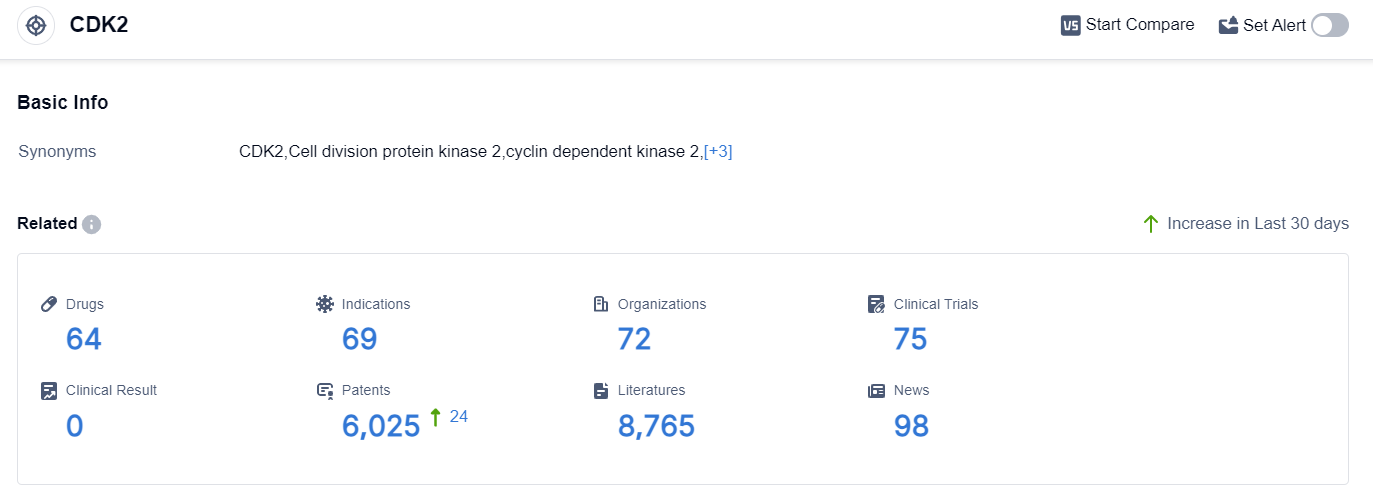
The analysis of the target CDK2 reveals that Merck KGaA, Pfizer Inc., and Cyclacel Pharmaceuticals, Inc. are the companies with the highest stage of development.
The drugs under the target CDK2 have been approved for indications such as B-Cell Chronic Lymphocytic Leukemia, Non-Small Cell Lung Cancer, Ovarian Cancer, and Triple Negative Breast Cancer. Small molecule drugs are progressing most rapidly, indicating intense competition in the market.
The United States, European Union, and several other countries are actively developing drugs targeting CDK2. Overall, the target CDK2 shows a competitive landscape with potential for future development in various indications and drug types.
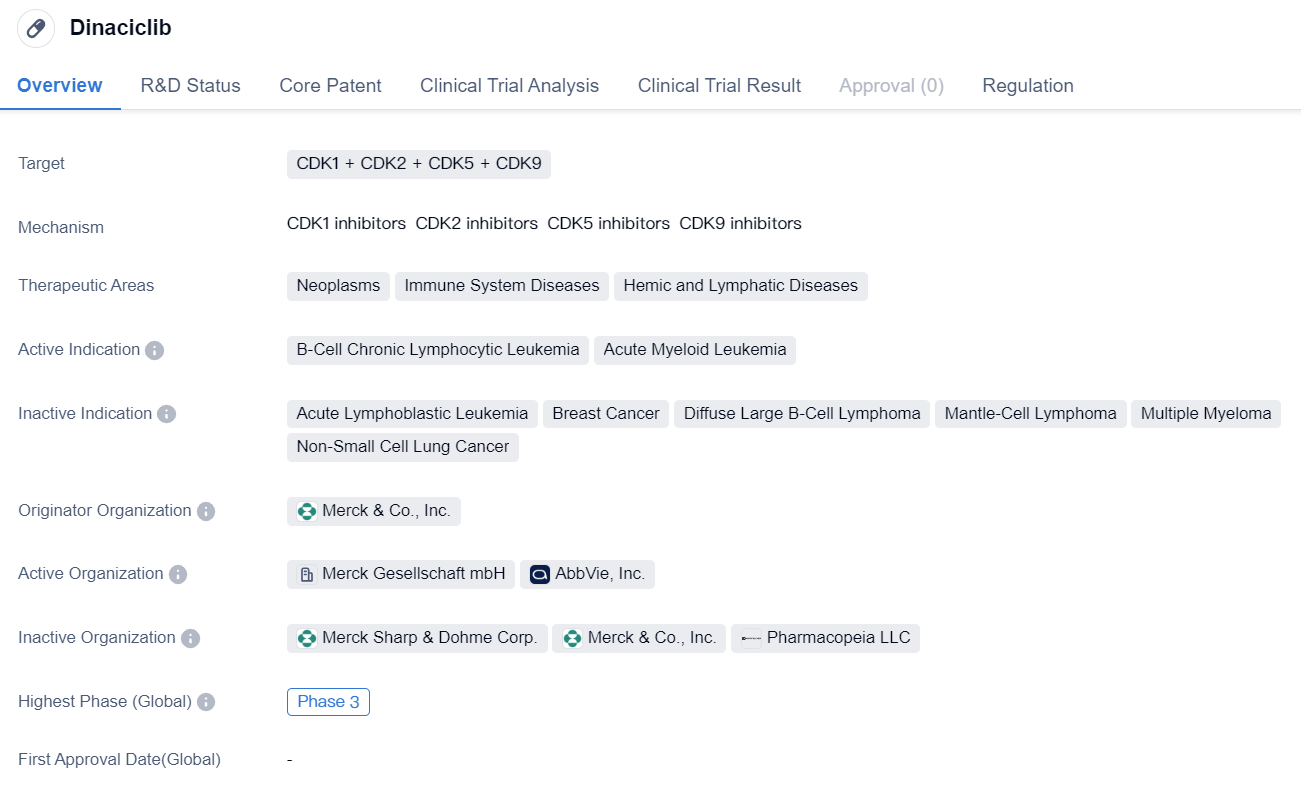
The multi-target CDK2 inhibitor Dinaciclib enters phase III clinical trials
Dinaciclib is a small molecule drug that targets multiple CDKs, including CDK1, CDK2, CDK5, and CDK9. It is being developed by Merck & Co., Inc. and is currently in Phase 3, which is the final stage of clinical trials before seeking regulatory approval.
The drug has shown potential therapeutic benefits in the treatment of neoplasms, immune system diseases, and hemic and lymphatic diseases. Specifically, it has demonstrated efficacy in B-cell chronic lymphocytic leukemia (B-CLL) and acute myeloid leukemia (AML). These indications are characterized by the abnormal growth and proliferation of certain cells in the blood and bone marrow.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Dinaciclib's mechanism of action involves inhibiting CDKs, which are enzymes involved in cell cycle regulation and transcriptional control. By targeting multiple CDKs, the drug aims to disrupt the abnormal cell growth and division observed in cancer and other diseases.
One notable aspect of Dinaciclib is its orphan drug status. Orphan drugs are medications developed to treat rare diseases or conditions that affect a small number of patients. This designation provides certain incentives and benefits to the drug developer, such as extended market exclusivity and financial support for clinical trials.
Overall, Dinaciclib holds promise as a potential treatment option for B-CLL and AML, two types of leukemia. Its small molecule nature and ability to target multiple CDKs make it an interesting candidate for further development. The ongoing Phase 3 trials will provide crucial data on its safety and efficacy, which will determine its potential for regulatory approval and commercialization.
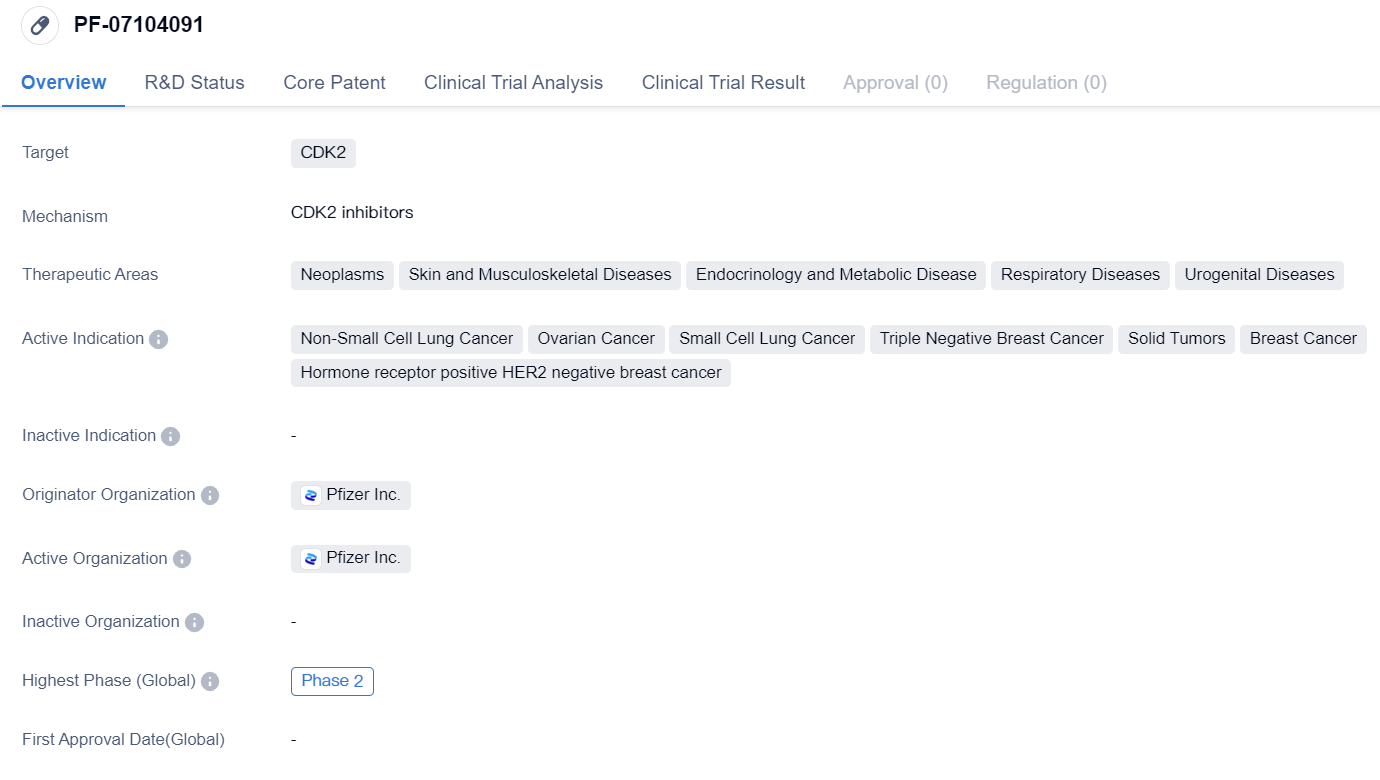
Selective CDK2 inhibitor PF-07104091 enters Phase II clinical trial
The drug PF-07104091 is a small molecule drug that targets CDK2, a protein involved in cell cycle regulation. It is being developed by Pfizer Inc., a leading pharmaceutical company.
PF-07104091 has shown potential in treating various therapeutic areas, including neoplasms (abnormal growth of cells), skin and musculoskeletal diseases, endocrinology and metabolic diseases, respiratory diseases, and urogenital diseases. It is specifically indicated for non-small cell lung cancer, ovarian cancer, small cell lung cancer, triple negative breast cancer, solid tumors, breast cancer, and hormone receptor positive HER2 negative breast cancer.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Currently, PF-07104091 has reached Phase 2 of clinical development globally. In China, it has reached Phase 1/2, indicating that it is still in the early stages of development in this region.
The objective presentation of this information allows stakeholders in the pharmaceutical industry to understand the key details of PF-07104091. The drug's small molecule nature suggests that it may have advantages in terms of formulation and delivery. Its target, CDK2, is a well-known protein involved in cell cycle regulation, making it a promising target for therapeutic intervention.
The wide range of therapeutic areas and indications for PF-07104091 indicates its potential for treating various diseases. Non-small cell lung cancer, ovarian cancer, and breast cancer are among the most prevalent and challenging cancers globally, making the drug's potential impact significant.
The fact that PF-07104091 has reached Phase 2 globally suggests that it has shown promising results in early clinical trials, demonstrating its safety and efficacy to a certain extent. However, its current phase in China, Phase 1/2, indicates that further research and development are needed to establish its effectiveness in this specific market.
In summary, PF-07104091 is a small molecule drug developed by Pfizer Inc. that targets CDK2. It has shown potential in treating various therapeutic areas and indications, particularly in the field of oncology. While it has reached Phase 2 globally, further development is required in China. This objective summary provides a concise overview of the drug's key details, allowing stakeholders to assess its potential and make informed decisions.