Brogidirsen - An antisense oligonucleotide targeting DMD exon 44
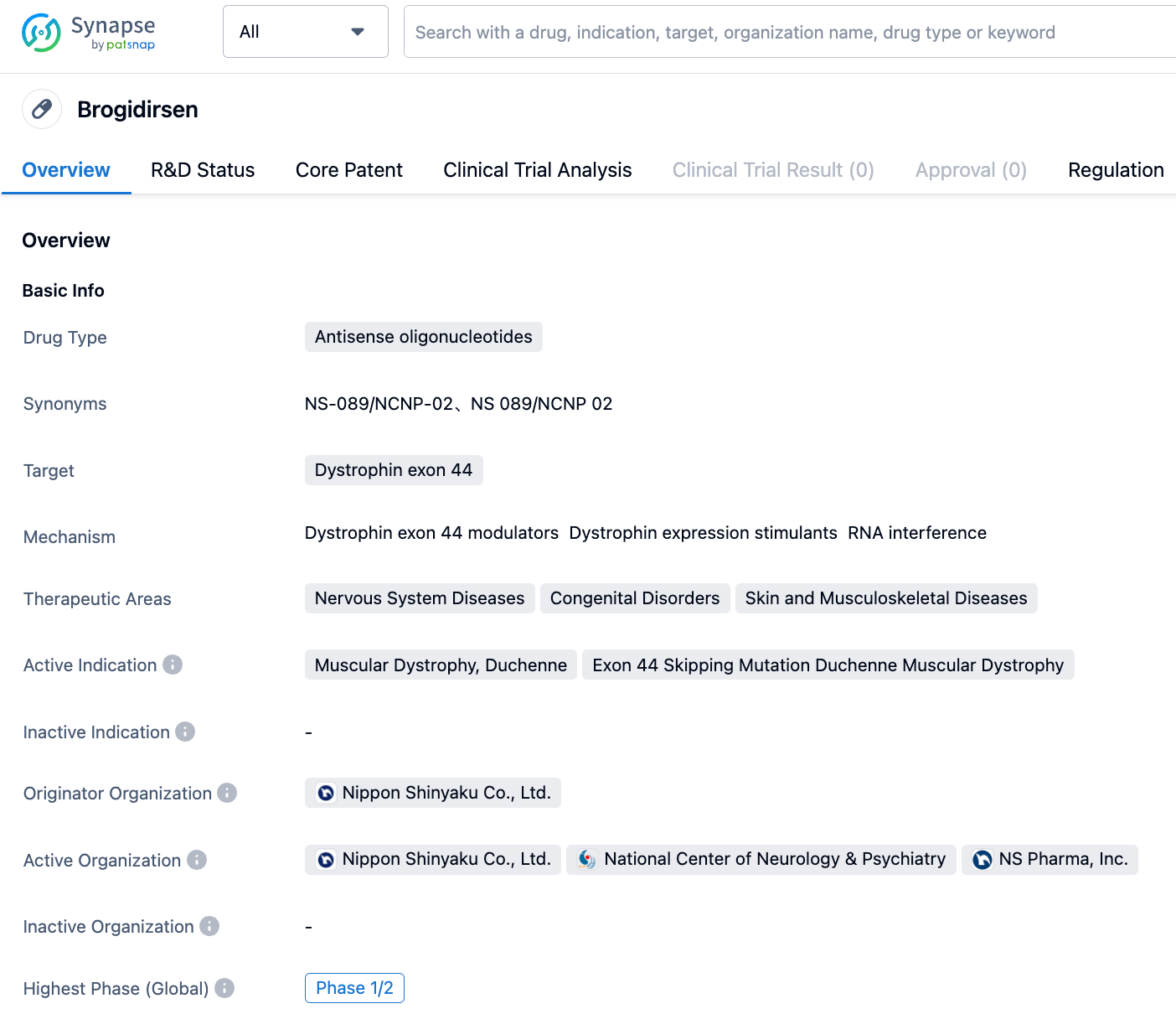
Brogidirsen is a drug targeting DMD exon 44 antisense oligonucleotide, developed by Nippon Shinyaku Co., Ltd. In July 2023, the drug was granted with three regulations by the FDA: Breakthrough Therapy, Orphan Drug, and Rare Pediatric Disease.
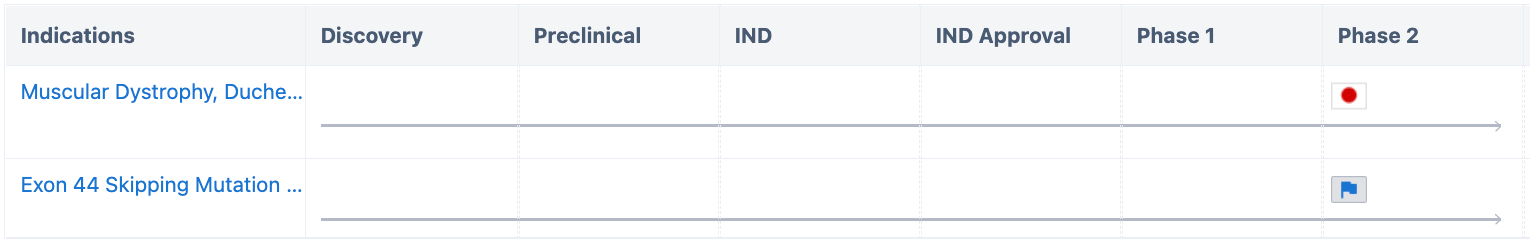
According to the "Synapse" database, the drug is currently under development for two indications, Duchenne Muscular Dystrophy (Duchenne) and Duchenne Muscular Dystrophy with exon 44 skipping mutation, both of which are in phase 2 clinical trials.
Despite being in phase 2, the drug has been granted multiple special review certifications. Its further developments are worth keeping an eye on.
Duchenne Muscular Dystrophy generally occurs in males and is a progressive form of muscular dystrophy, this disease leads to weakening and loss of function in skeletal muscle, heart muscle, and respiratory muscles. The early signs of Duchenne can include delays in the ability to sit, stand, or walk. It is a continually progressing disease, and patients may need a wheelchair in their adolescence. Problems with cardiac and respiratory muscles can emerge in the teenage years, leading to severe, potentially life-threatening complications.
Mechanism of Action
DMD exon 44 typically refers to a specific region linked to mutations of the DMD gene. Mutations in DMD exon 44 can cause errors in the creation process of dystrophin, thus preventing the production of normal dystrophin. Some targeted drugs aim to "skip" this mutated exon, enabling cells to bypass this error and continue to create partially functioning dystrophin. This strategy, known as "exon skipping," may help to alleviate the symptoms of the disease but is currently still in the clinical trial phase.
Competitive Landscape
There are three other drugs in global development that target Dystrophin exon 44, including AOC-1044, developed by Avidity Biosciences, Inc., which is also in phase 1/2 clinical trials. The others are either in the discovery phase or their development has already halted.