Fruquintinib - A Small Molecule Drug Targeting VEGFR1/VEGFR2/VEGFR3
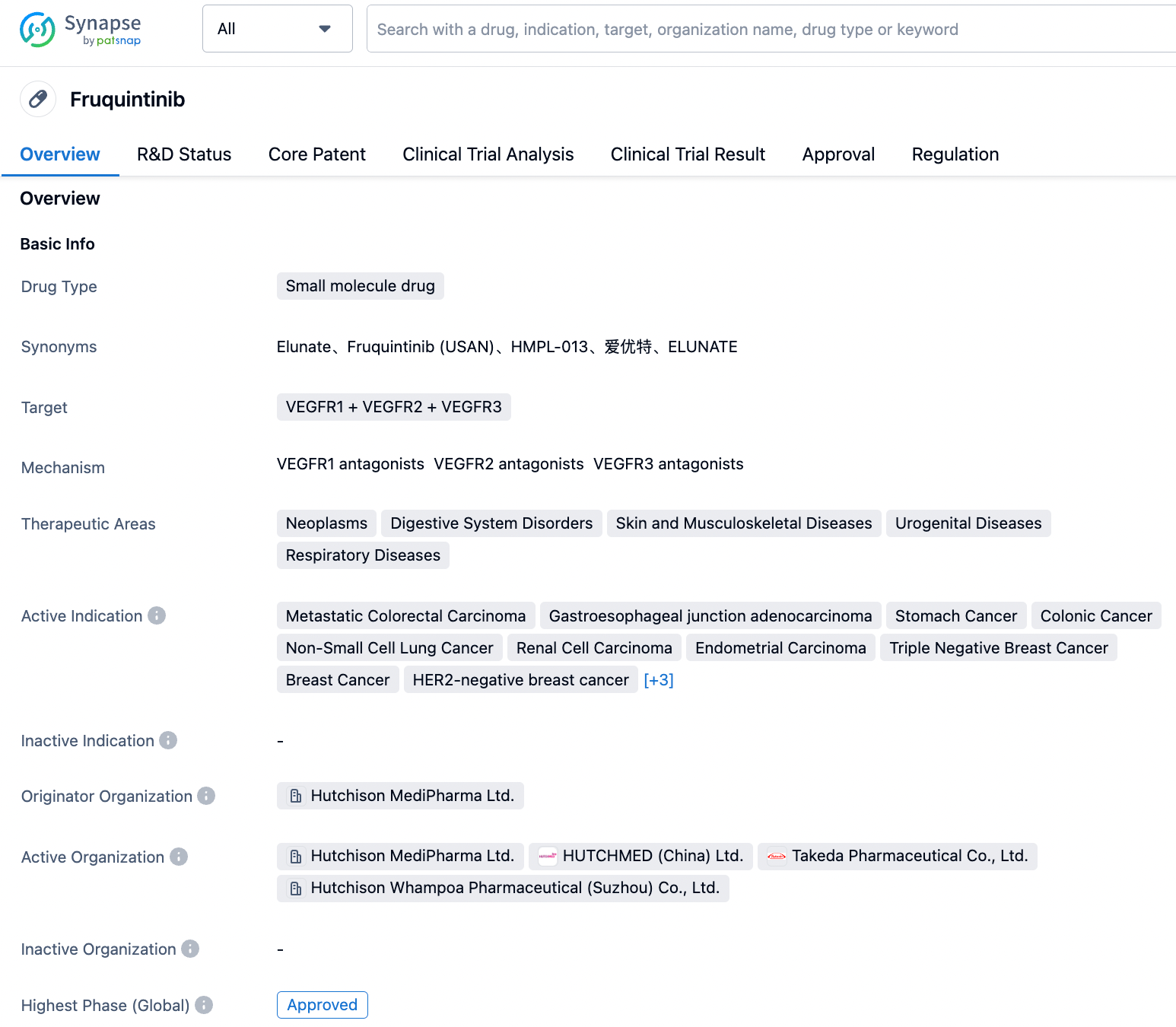
Fruquintinib is a small molecule drug targeting VEGFR1, VEGFR2, and VEGFR3. This drug was initially developed by Hutchison Whampoa Medicine (Shanghai) Co., Ltd. On January 23, 2023, it entered into an exclusive licensing agreement with a subsidiary of Takeda Pharmaceutical.
Takeda Pharmaceutical will obtain the exclusive global licensing for all indications of Fruquintinib, excluding mainland China, Hong Kong, and Macau. Hutchison Whampoa will receive a payment of up to $1.13 billion (with a down payment of $400 million).
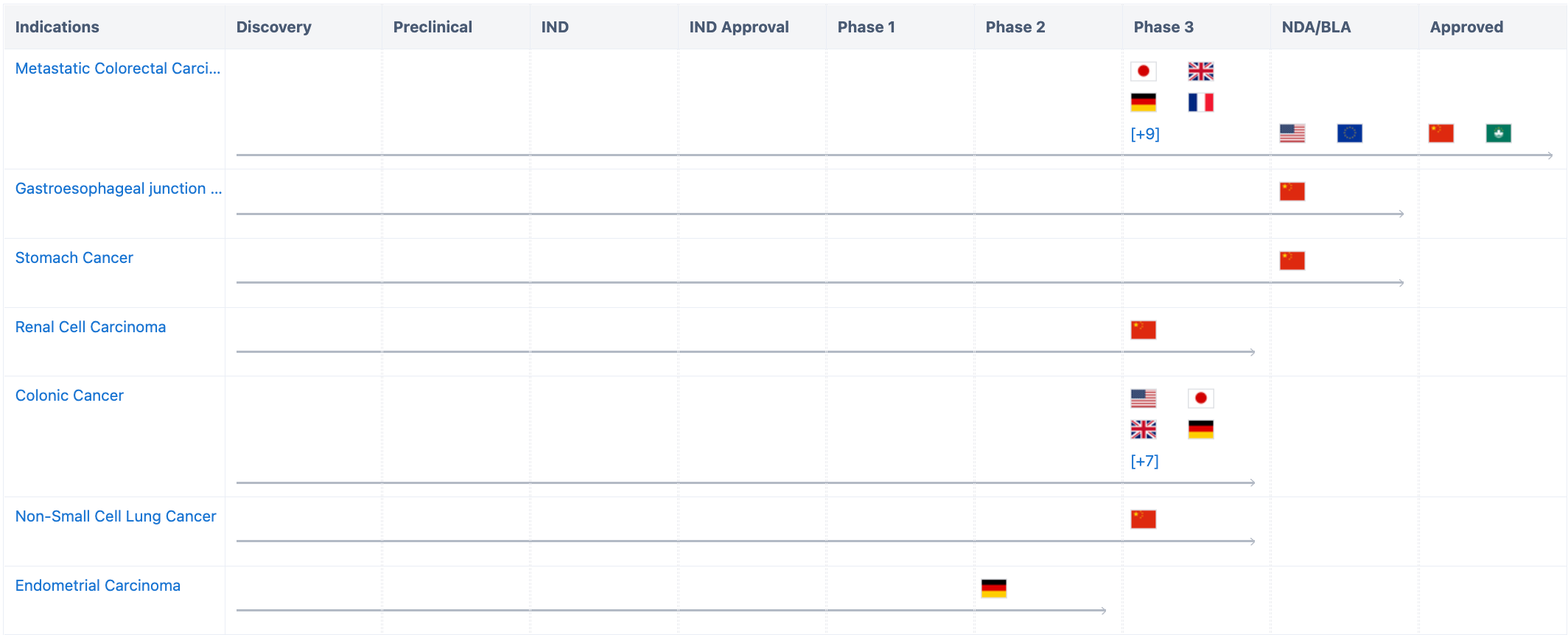
This drug was first approved in China on September 4, 2018, for the treatment of metastatic colorectal cancer patients who had previously undergone treatment with fluoropyrimidine, oxaliplatin, and irinotecan. It was approved in Macau, China on March 1, 2022. On July 10, 2023, for endometrial cancer, the NMPA included Fruquintinib in breakthrough therapy certification.
According to the "Synapse" database, At present, the indications for this drug include metastatic colorectal cancer, adenocarcinoma of the gastroesophageal junction, stomach cancer, colon cancer, non-small cell lung cancer, renal cell carcinoma, endometrial cancer, triple-negative breast cancer, breast cancer, HER2-negative breast cancer, HR-positive breast breast cancer, metastatic breast cancer, rectal cancer, etc. Among them, metastatic colorectal cancer has not only been approved in China but has also filed for listing in Europe and the US, and is in Phase 3 of clinical trials in Japan, the UK, Germany, and 13 other countries. A Phase 3 clinical trials are currently conducted in patients with endometrial cancer.
In addition to the breakthrough therapy certification from NMPA this time, as early as 2017 and 2020, it was granted priority review (China) and fast track (USA) certifications for colorectal cancer. In May this year, it was granted priority review (USA) for metastatic colorectal cancer.
Core Clinical Trials
For refractory colorectal cancer: In the phase 3 clinical study (NCT04322539, FRESCO-2), the overall survival (OS) of the Fruquintinib + Best Supportive Care (BSC) group was 7.4 months, while for the placebo + BSC group, it was 4.8 months.
For metastatic colorectal cancer: In the phase 3 clinical study (NCT02314819, FRESCO), the CEA response rate within the Fruquintinib group was 30.0%, while in the placebo group it was 1.3%. The OS of the Fruquintinib group was 9.3 months, while the OS of the placebo group was 6.6 months. The results of these two studies further support the potential value of Fruquintinib in the treatment of refractory tumors.