FCN-159 - An Oral High-Efficiency Selective MEK1/2 Inhibitor
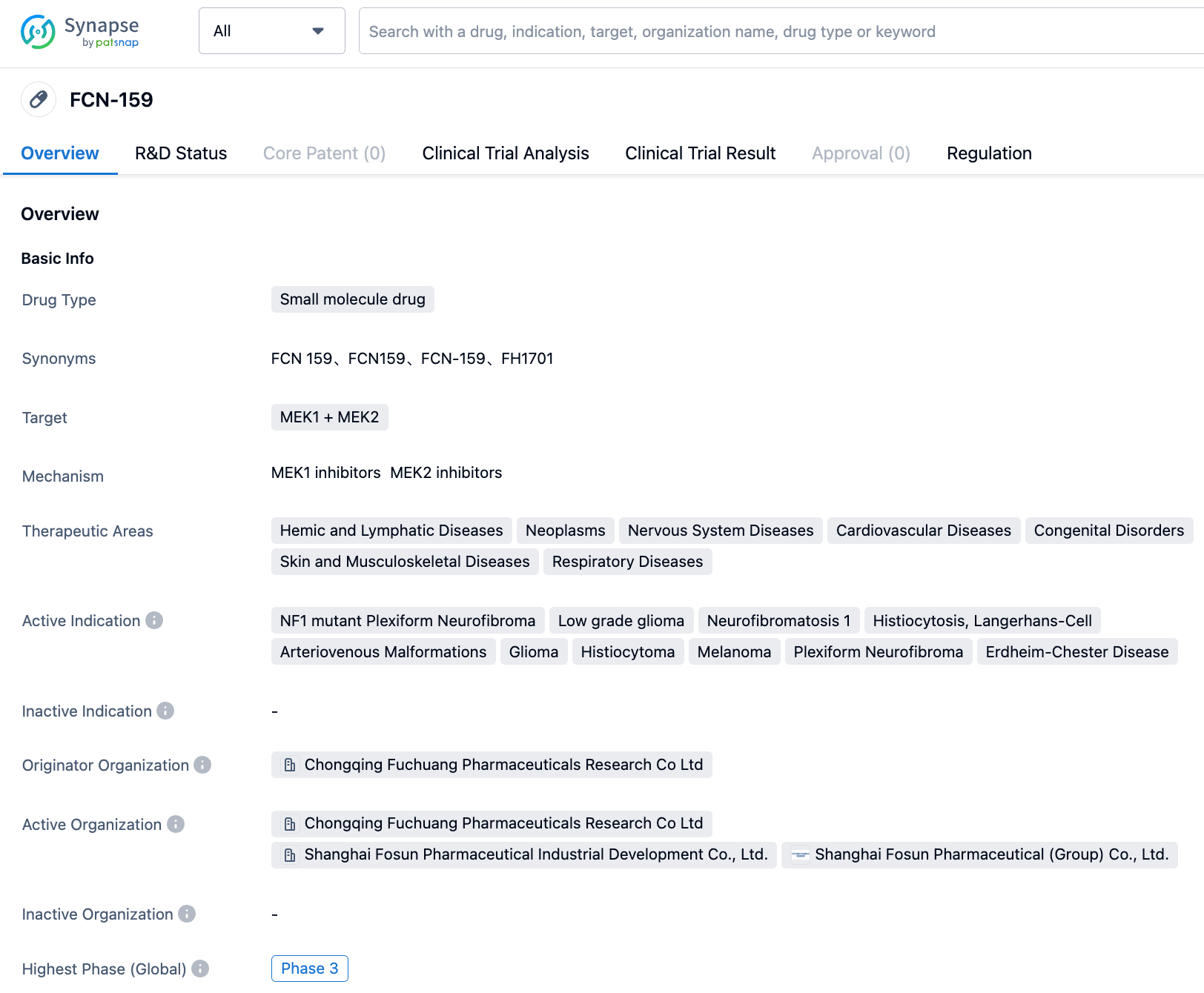
FCN-159 is an orally effective selective MEK1/2 inhibitor and a prospective targeted therapy drug for tumors mutated by BRAF or RAS. Its selectivity is ten times higher than that of Trametinib. Fosun Pharma's subsidiary is currently developing multiple indications.
On April 13, 2023, for the first time, it was awarded the NMPA Breakthrough Therapy Designation for histiocytoma. Then again, on July 17, 2023, it has been granted the NMPA Breakthrough Therapy Designation for NF1 mutated plexiform neurofibromas. In the future, FCN-159 is likely to be launched, offering new treatment options and hope for patients.
R&D Status
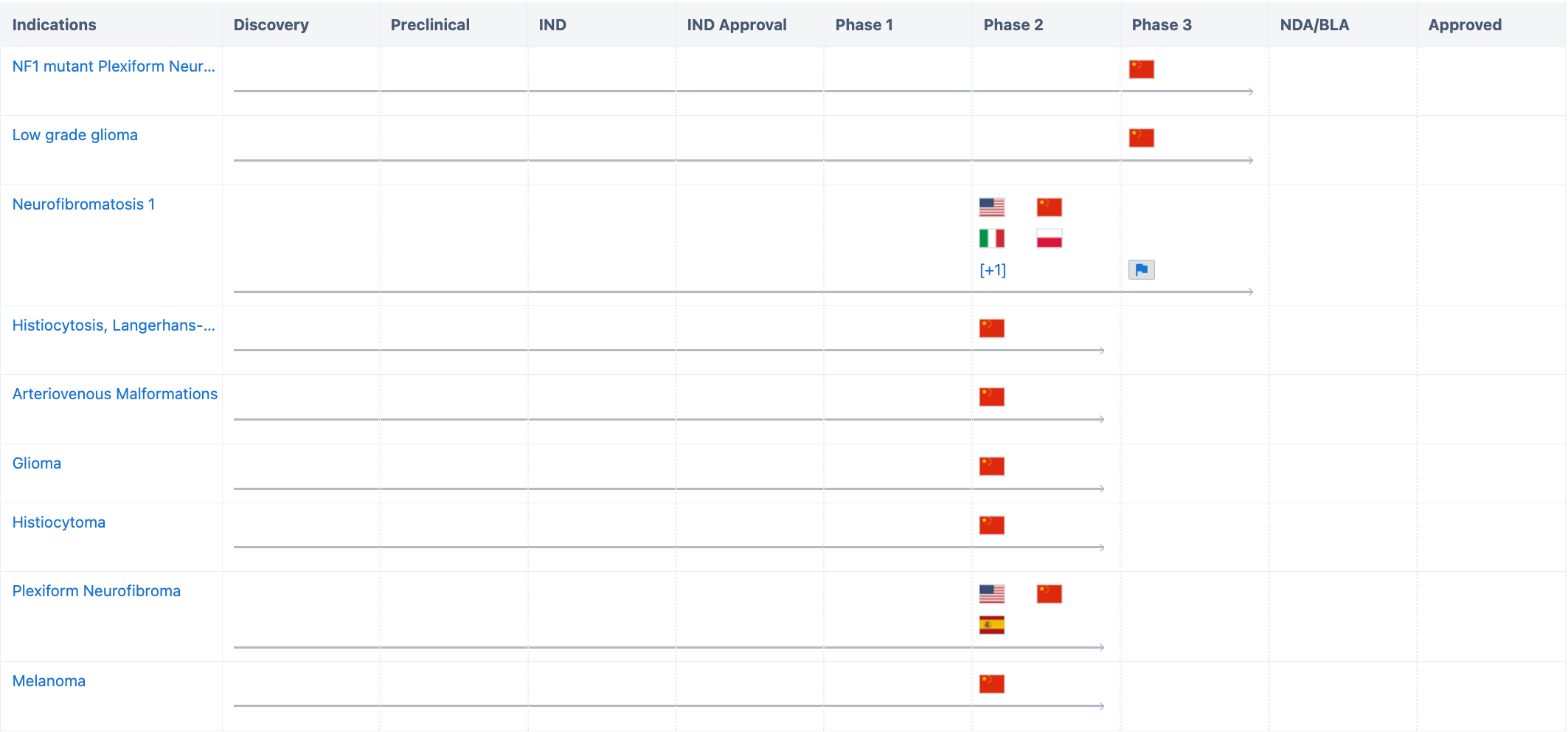
According to the "Synapse" database, The current indications include NF1 mutant plexiform neurofibromas (Neurofibromatosis type 1), low-grade gliomas, arteriovenous malformations, etc., all of which have entered the clinical trial stage. Among them, the most advanced in R&D are NF1 mutant plexiform neurofibromas and low-grade gliomas, which have currently entered Phase 3 clinical trials in China. Neurofibromatosis type 1 is conducting clinical trials in multiple countries around the world and has already entered Phase 2 clinical trials in the United States, China, Italy, Spain, and Poland. In addition, research related to arteriovenous malformations, gliomas, histiocytomas, melano-mas, plexiform neurofibromas has already entered Phase 2 clinical trials.
In addition to NF1 mutant Plexiform Neurofibroma, Histiocytoma was also granted Breakthrough Therapy (CN) on April 13, 2023.
NF1 mutant Plexiform Neurofibroma is an autosomal dominant genetic disease caused by a mutation in the NF1 gene. Normally, the pro-tein expressed by this gene can inhibit cell growth and division. However, when the gene mutates, the expressed protein loses its inhibi-tory function, leading to a series of symptoms, including unevenly-sized dark spots on the skin, and the growth of neurofibromas. Patients typically start showing symptoms during childhood.
Core Clinical Trials
Neurofibromatosis Type 1: In the phase 1/2 clinical trial NCT04954001, a total of 82 subjects received FCN-159 treatment. The primary endpoint was ORR, and secondary endpoints were the incidence of serious adverse events and treatment-related adverse events. The results showed an ORR of 31.7% (95% Confidence Interval: 21.9%~42.9%). The occurrence rate of serious adverse events was 1.2%. The proportion of adverse events needing treatment discontinuation was 12.2%, and the proportion requiring dose reduction was 9.8%. The incidence rates of treatment-related adverse events of grade 3 or above were: folliculitis 25.6%, periungual inflammation 4.9%.
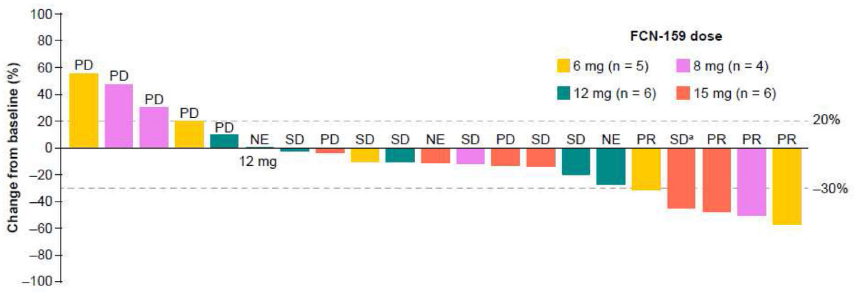
Advanced NRAS-mutant melanoma: Thirty-three patients were enrolled across nine FCN-159 dose groups (0.2-15 mg QD). One DLT occurred: grade 3 folliculitis in the 15-mg group. There was one grade > 3 treatment-emergent adverse event (TEAE), death of unknown aetiology (not FCN-159 related). The most common FCN-159-related TEAE was rash (36.4%), and the incidence of grade ≥3 FCN-159-related TEAEs was 15.2%. Antitumour activity at QD doses < 6 mg was limited; therefore, efficacy data are presented only for doses ≥6 mg (n = 21). The objective response and clinical benefit rates were 19.0% (four partial responses) and 52.4%, respectively. Median (95% confidence interval) duration of response and progression-free survival were 4.8 months (2.8-not reached) and 3.8 months (1.8-5.6), respectively. FCN-159 exposure increased dose-proportionately; geo-metric mean terminal half-life was 29.9-56.9 h.