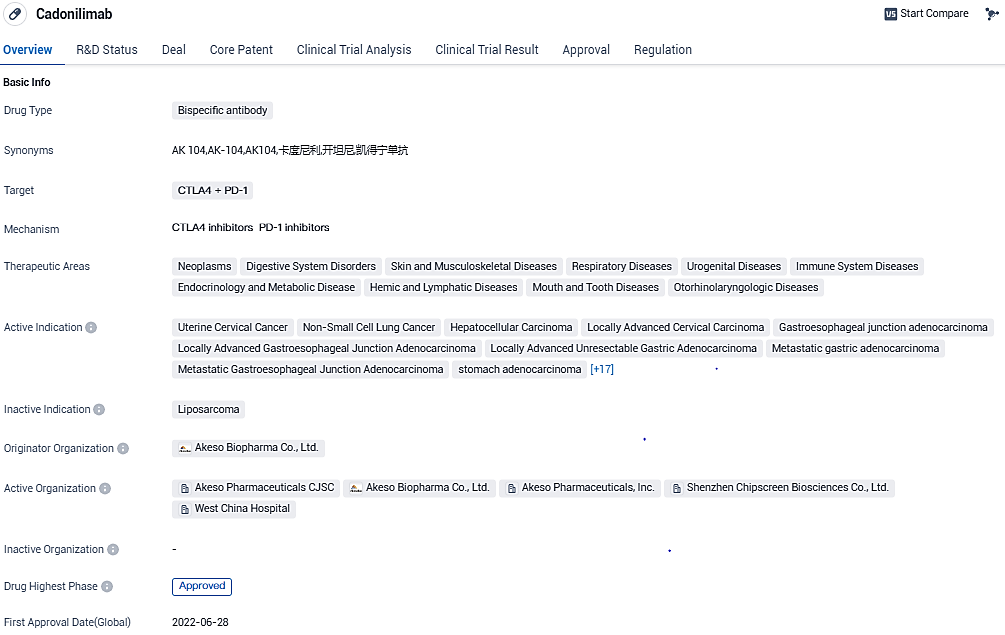
Cadonilimab, a PD-1/CTLA-4 bispecific antibody, met its primary endpoint by halting progression in a Phase III recurrent/metastatic cervical cancer trial
Akeso reported that the Phase III AK104-303 trial, testing their PD-1/CTLA-4 bispecific antibody cadonilimab, in combination with platinum-based chemotherapy, with or without bevacizumab, has reached one of its main objectives of PFS in the initial treatment of recurring or metastasized cervical cancer. The test presented a p-value of under 0.0001.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The commencement of the AK104-303 trial is a pivotal event, being the first Phase III clinical examination to test the pairing of a PD-1/CTLA-4 bispecific antibody with platinum-based chemo +/- bevacizumab for initial treatment versus relapse/metastatic R/M CC. Cadonilimab, in combination with others versus placebo, witnessed a promising trend in the enhancement of overall survival, another main trial endpoint. OS data was not final at the interim analysis, however, the trial would proceed to evaluate OS.
Securing the main trial endpoint of PFS in the Phase III trial of cadonilimab for initial cervical cancer therapy sets another significant benchmark for the drug in frontline treatment. This follows previous success in hitting the primary endpoint for gastric cancer in the first line, leading the way for planned sNDA by Akeso.
In the middle of 2022, cadonilimab got approval for the second and third-line therapy for advanced cervical cancer. With promising results in the drug's registration trials across unique indications, particularly first-line therapies, a larger patient group eligible for cadonilimab is expected to quickly increase. This surge would more effectively utilize the clinical benefits of this innovative bispecific immuno-oncology therapy, extending its reach to a bigger patient base.
Dr. Yu Xia, the founder, CEO, Chairman, and President of Akeso said, "Once again, we're thrilled to see the significant amelioration in progression-free survival realized with cadonilimab in the primary treatment of all patients with advanced cervical cancer."
He expressed his deepest gratitude to all the research staff, participants, and patients who took part in this trial. "It's because of your commitment and hard work that more cervical cancer patients in China will have the chance to benefit from this revolutionary IO therapy, improving treatment efficacy and survival rates," Dr. Xia added.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
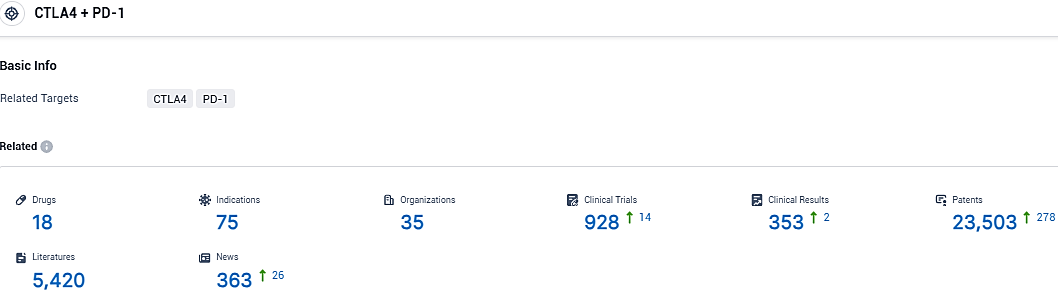
According to the data provided by the Synapse Database, As of November 30, 2023, there are 18 investigational drugs for the PD-1/CTLA-4 target, including 75 indications, 35 R&D institutions involved, with related clinical trials reaching 928, and as many as 23503 patents.
Cadonilimab's approval in China in 2022 marks a significant milestone for Akeso Biopharma Co., Ltd. and demonstrates the company's commitment to advancing innovative therapies in the field of biomedicine. The drug's bispecific antibody mechanism of action, targeting both CTLA4 and PD-1, offers a unique approach to modulating the immune system and combating cancer.