Exploring Tebotelimab 's R&D successes and its clinical results at the 2023 AACR
The latest clinical findings of Tebotelimab, a PD-1/LAG-3 bispecific antibody, in patients with untreated, unresectable, recurrent or metastatic, mucosal melanoma were unveiled at the 2023 AACR Congress, demonstrating its potential clinical effect.
Tebotelimab's R&D Progress
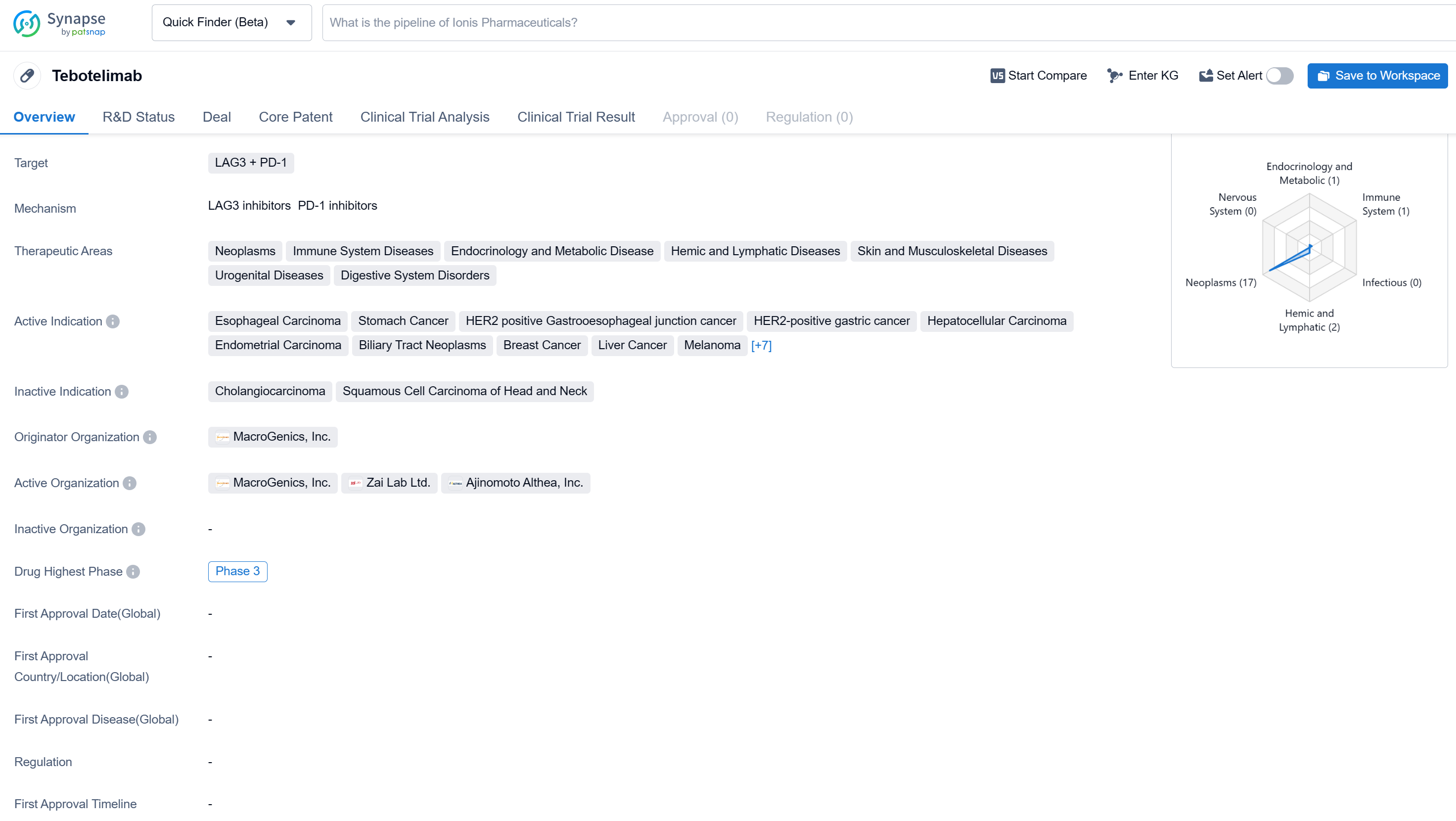
Tebotelimab is a bispecific antibody drug that targets LAG3 and PD-1. It falls under the therapeutic areas of neoplasms, immune system diseases, endocrinology and metabolic disease, hemic and lymphatic diseases, skin and musculoskeletal diseases, urogenital diseases, and digestive system disorders.
According to the Patsnap Synapse, Tebotelimab has reached the highest phase of clinical development, which is Phase 3 globally. In China, the highest phase of development is Phase 2/3. And the clinical trial areas for Tebotelimab are primarily in the United States, China, and Unite Kingdom. The key indication is Squamous Cell Carcinoma of Head and Neck. 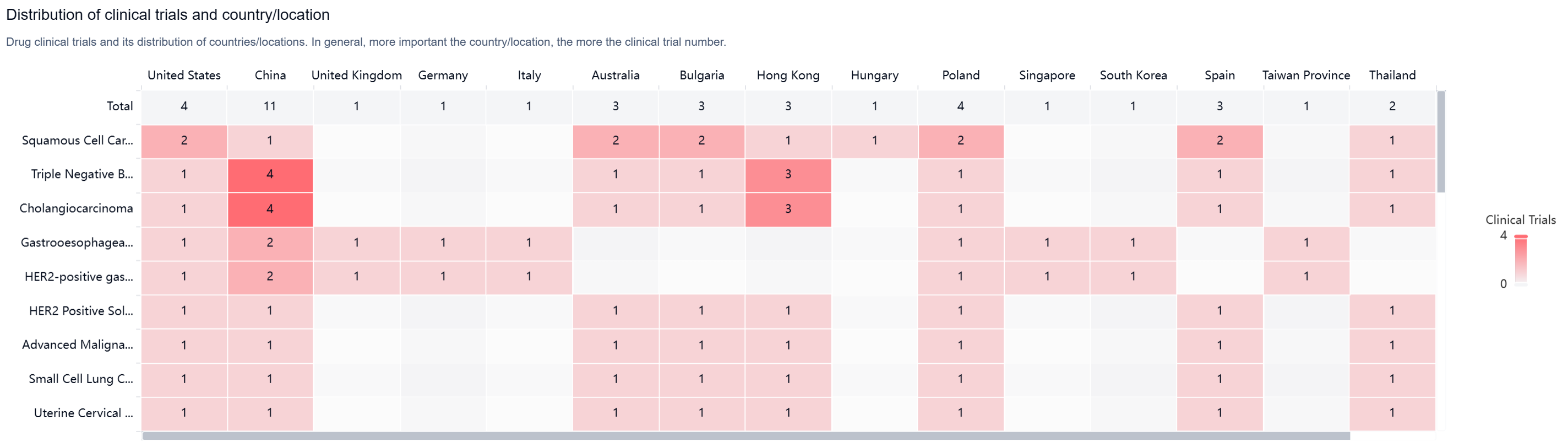
Detailed Clinical Result of Tebotelimab
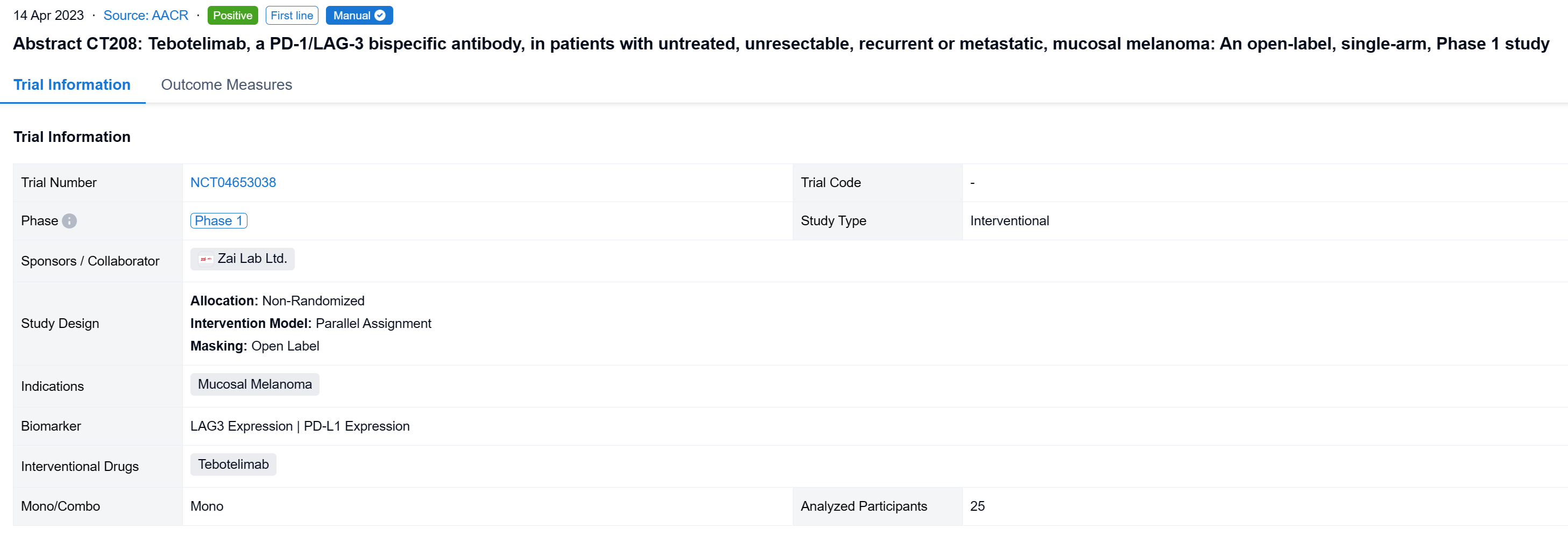
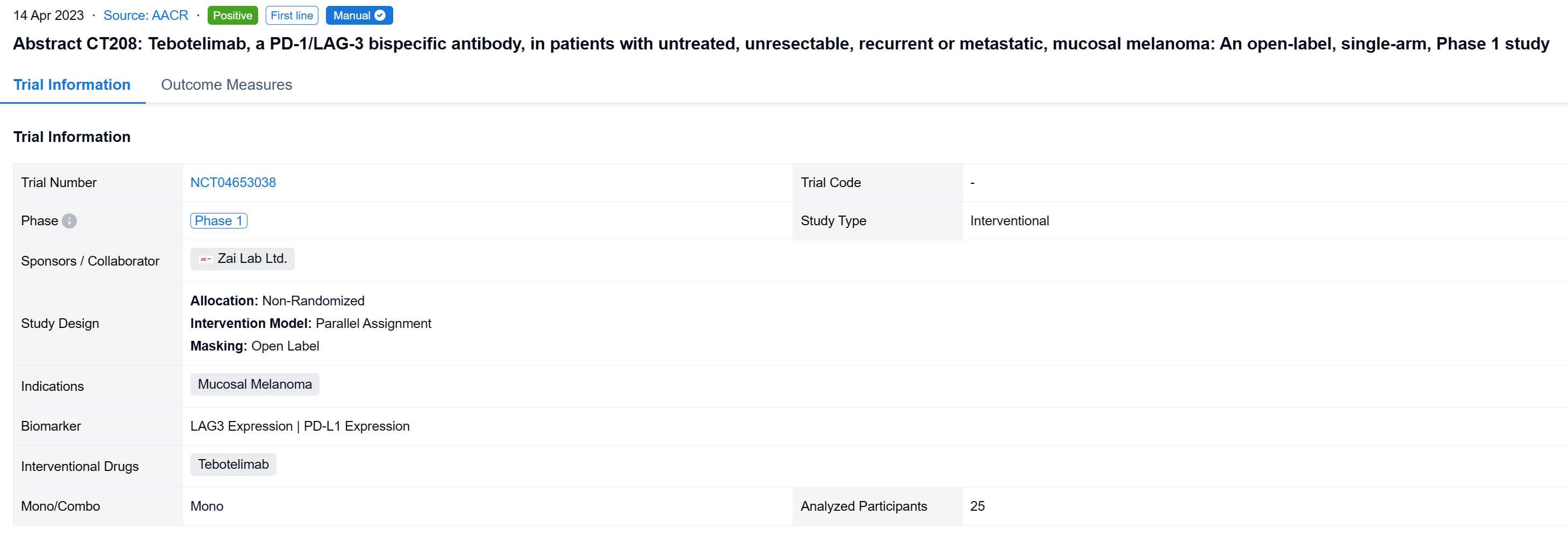
The non-randomized, parallel assignment, open-labeled clinical trial (NCT04653038) to assess the efficacy and safety of tebotelimab in melanoma patients (pts) including those with CPI-naïve mucosal melanoma.
In this study, the CPI-naïve cohort of this study enrolled pts with unresectable, recurrent or metastatic, mucosal or acral melanoma who had received no systemic therapy. Tebotelimab 600 mg was administered intravenously once every two weeks. The primary endpoint was overall response rate (ORR) assessed by independent radiologic review committee (IRC) per RECIST v1.1 in the efficacy analysis set consisting of pts who received ≥1 dose of tebotelimab. A post-hoc sensitivity analysis was conducted in the IRC-response evaluable set consisting of pts with IRC-assessed target lesions in the efficacy analysis set who received ≥1 post-baseline tumor assessment by IRC or died within 13 weeks after first dose. Results are reported for mucosal melanoma.
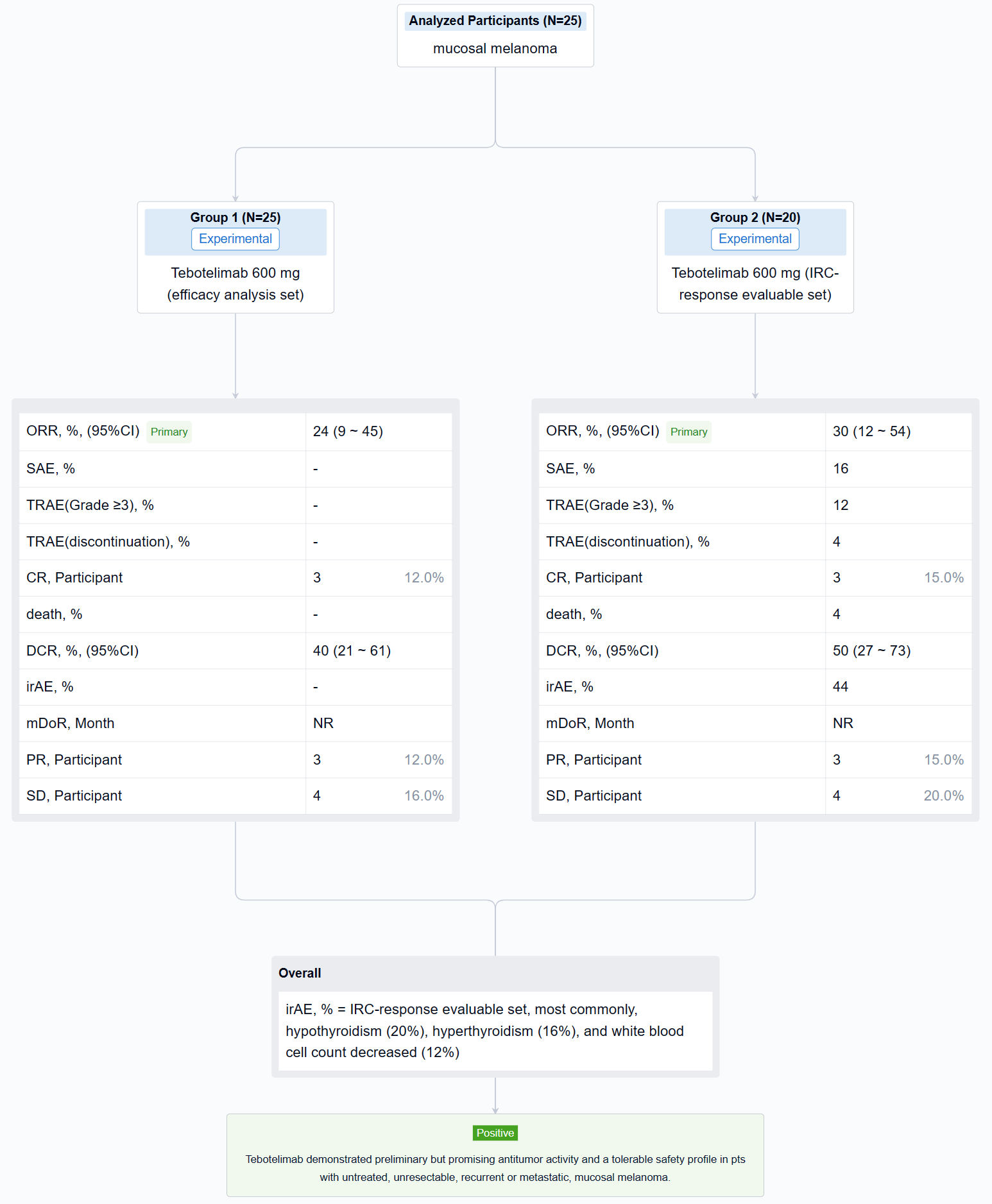
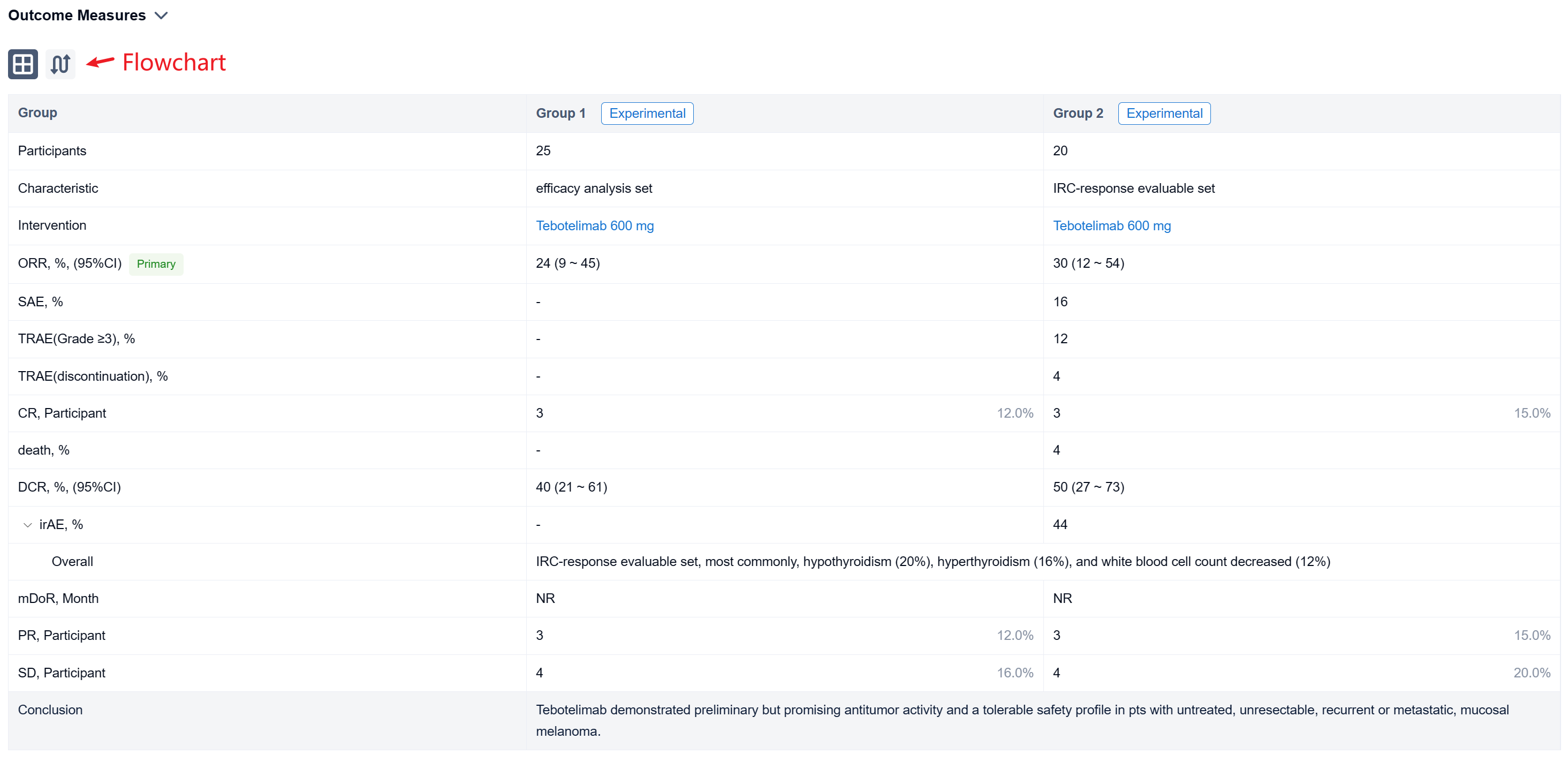
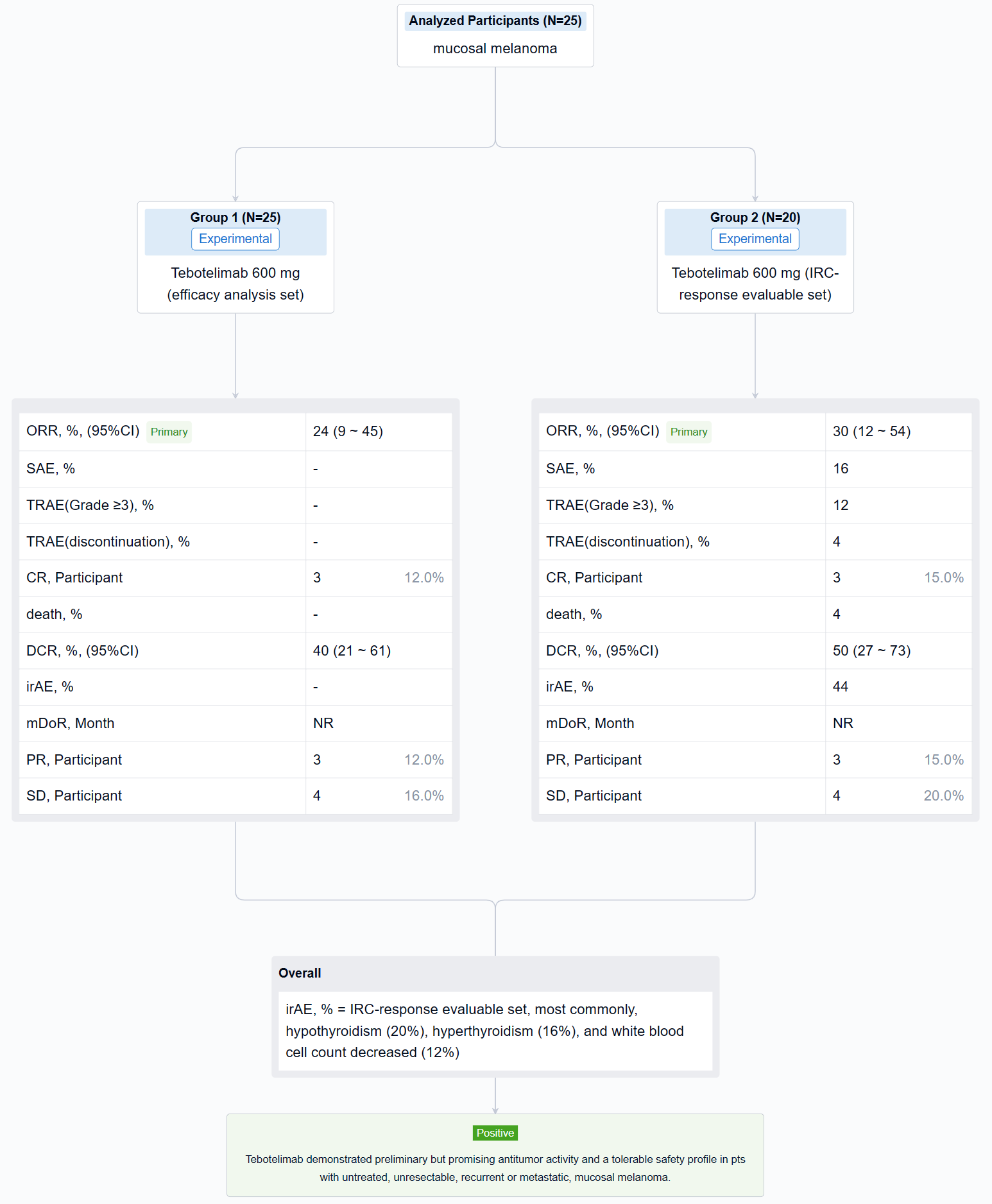
The result showed that at data cut-off (January 19, 2022), 25 pts with mucosal melanoma were enrolled (median age, 61 years; male, 40%; ECOG 1, 40%; TNM Stage IV, 92%; metastatic, 80%). LAG-3 expression level was ≥1% in seven (28%), <1% in 15 (60%), and unknown in three (12%). PD-L1 expression was positive (CPS≥1) in three (12%), negative (CPS<1) in 19 (76%), and unknown in three (12%). All pts received ≥1 dose of tebotelimab. In the efficacy analysis set (n=25), three, three, and four pts achieved complete response (CR), partial response (PR), and stable disease (SD), respectively, leading to a confirmed ORR of 24% (95% confidence interval [CI], 9-45), with median duration of response (DOR) not reached, and a disease control rate (DCR) of 40% (95% CI, 21-61). In the IRC-response evaluable set (n=20), three, three, and four pts achieved CR, PR, and SD, respectively, leading to a confirmed ORR of 30% (95% CI, 12-54), with median DOR not reached, and a DCR of 50% (95% CI, 27-73). Immune-related treatment-emergent adverse events occurred in 11 (44%) pts, most commonly, hypothyroidism (20%), hyperthyroidism (16%), and white blood cell count decreased (12%). Grade ≥3 and serious treatment-related adverse events (TRAEs) were reported in three (12%) and four (16%) pts, respectively. TRAEs led to treatment discontinuation and death each in one (4%).
It can be concluded that Tebotelimab demonstrated preliminary but promising antitumor activity and a tolerable safety profile in pts with untreated, unresectable, recurrent or metastatic, mucosal melanoma.
How to Easily View the Clinical Results Using Synapse Database?
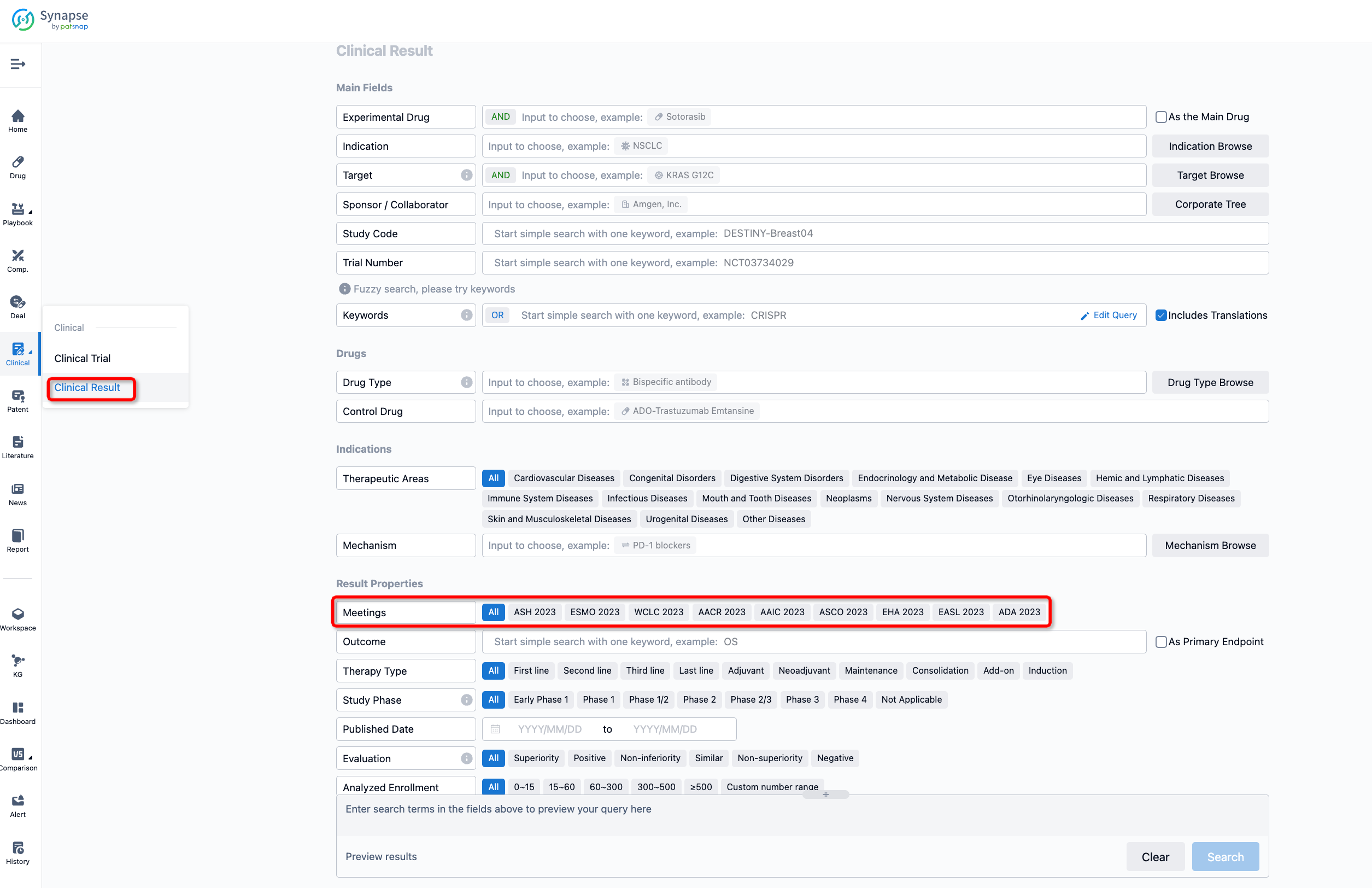
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
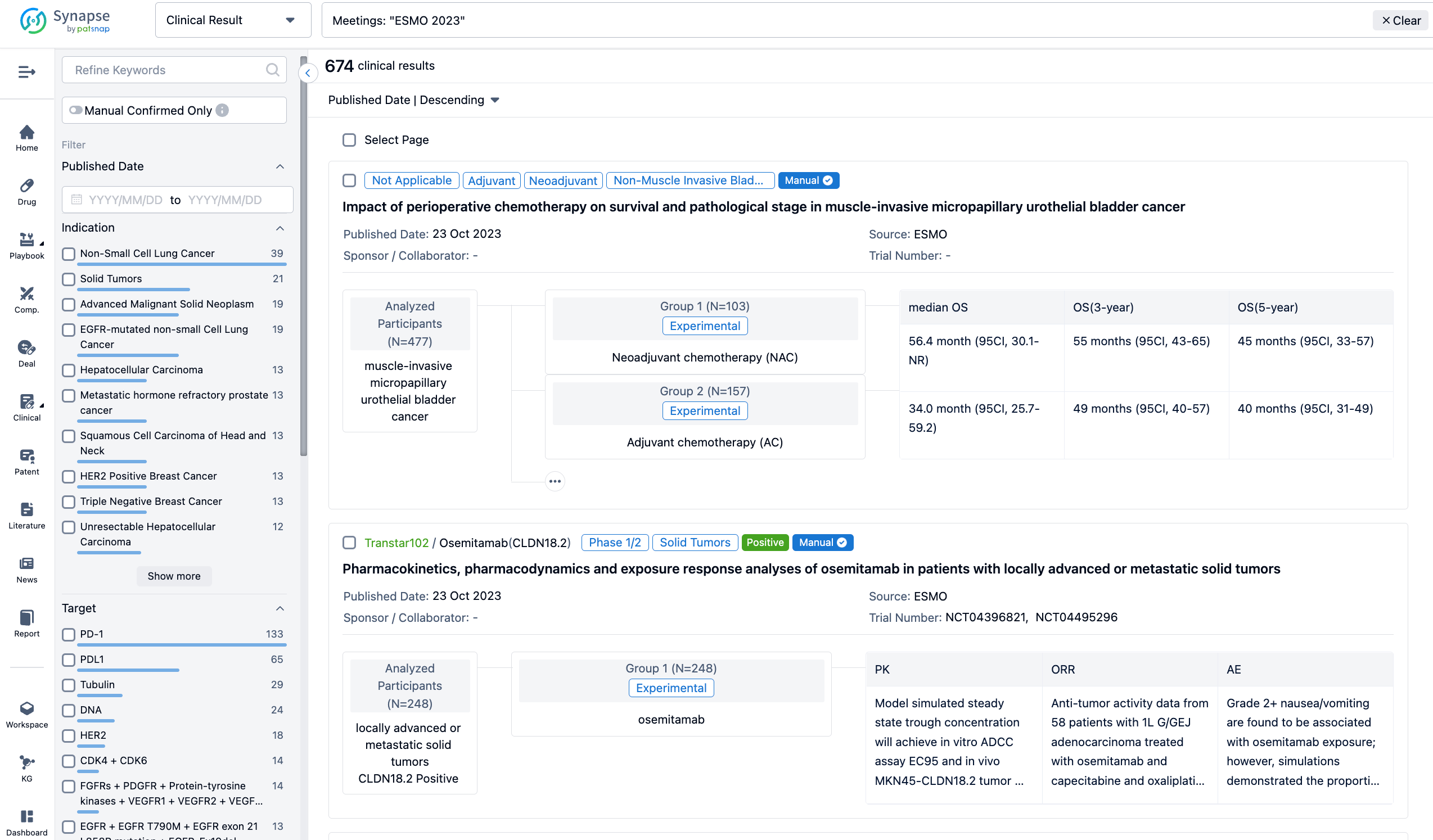
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
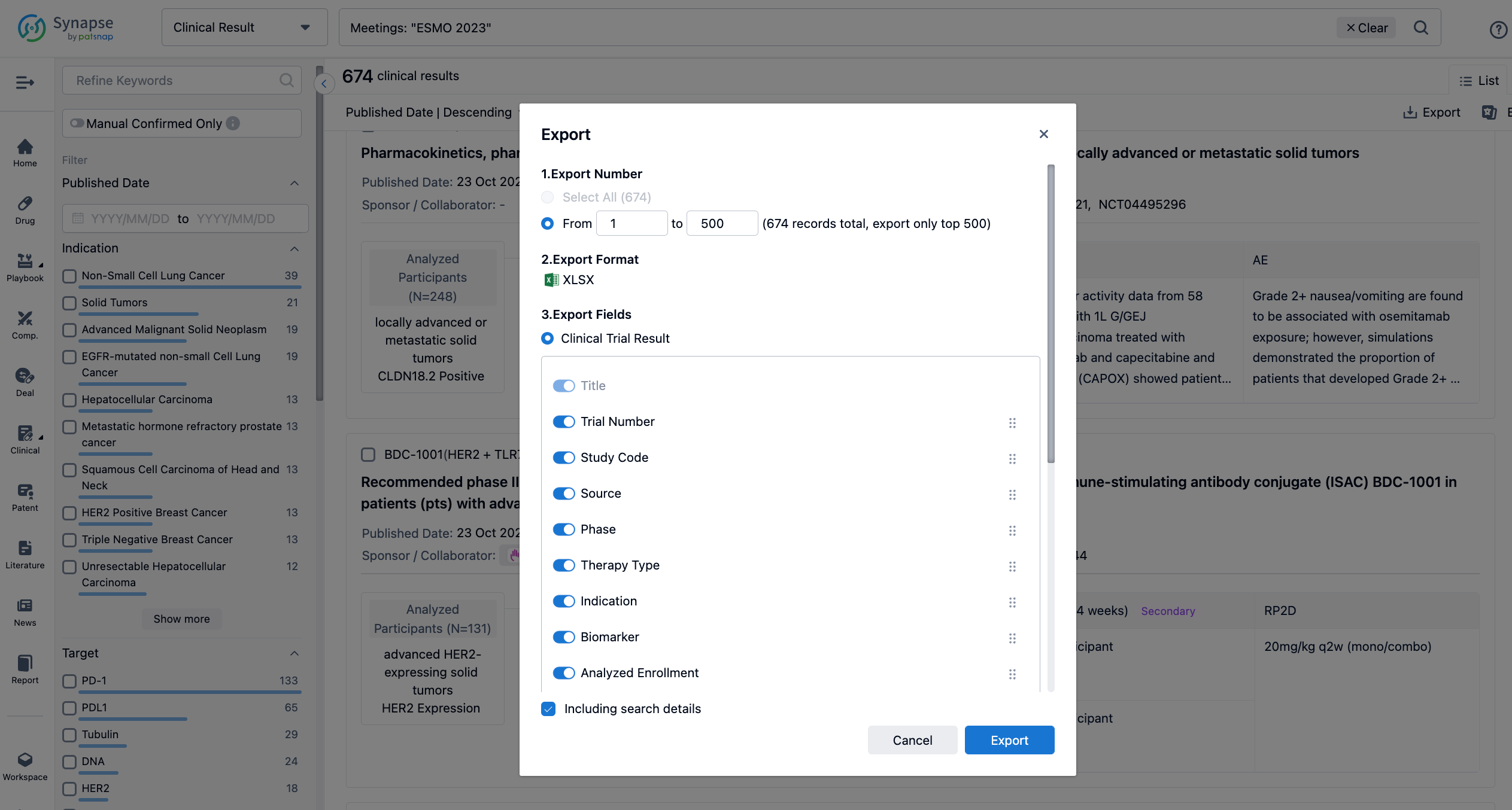
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!