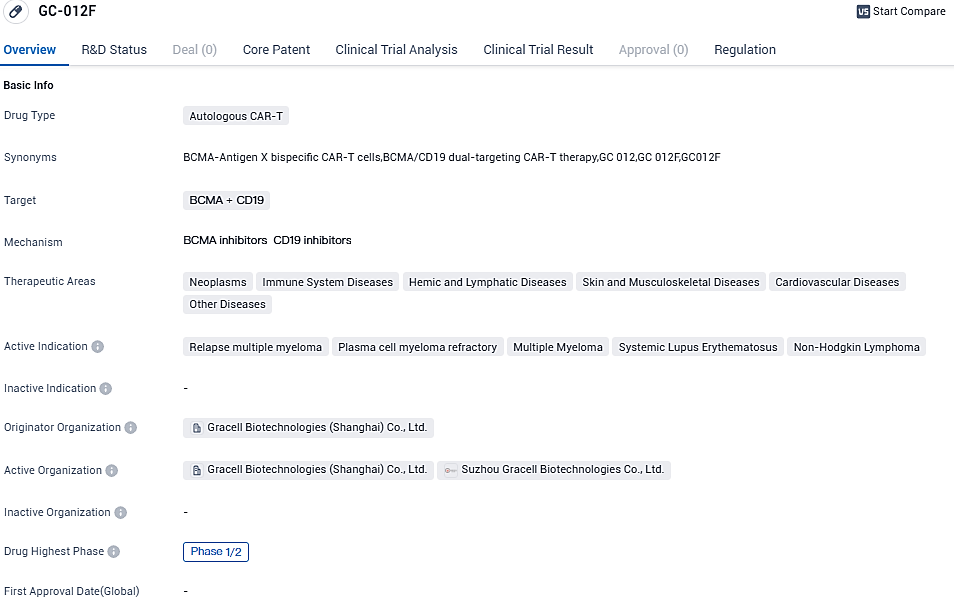
Gracell Biotechnologies announces FDA clearance for a Phase 1/2 trial of FasTCAR-T GC012F for treatment-resistant Systemic Lupus Erythematosus
FDA has given the green light for Gracell Biotechnologies Inc. to commence Phase 1/2 clinical trials of FasTCAR-T GC012F in America. The worldwide biopharma firm focuses on creating advanced, effective cell therapies to combat cancer and autoimmune disorders, particularly refractory systemic lupus erythematosus.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"We enthusiastically look forward to increased clinical progress in our top FasTCAR property, GC012F, explicitly aimed at treating rSLE within the USA," stated Dr. William Cao, the creator, Chairman, and Central Officer of Gracell. “As a cutting-edge CAR-T medical procedure, GC012F joins the novel double aimed CD19/BCMA strategy with our revolutionary FasTCAR next-day manufacturing technology. Both elements can likely offer substantial benefits to SLE patients."
The GC012F is an autologous CAR-T medical candidate with a double target strategy against BCMA and CD19, based on Gracell's unique FasTCAR overnight manufacturing platform. Besides the impending rSLE IND examination, GC012F is undergoing evaluation in the 1b/2 IND phase to treat relapsed or resistant multiple myeloma in the USA and in four IIT trials to treat rSLE, RRMM, newly-diagnosed multiple myeloma, and B-NHL.
Systemic lupus erythematosus is an autoimmune disease caused by B-cells, wherein the patient's immune system creates antibodies that attack their own body tissues, resulting in damage to multiple organs. Despite using immunosuppressants as the accepted standard of care, handling SLE remains a challenge. As a chronic condition, it heavily affects the quality of life and currently, there is no known cure. There is a desperate, unfulfilled medical necessity for more efficient and possibly curative treatments, especially suitable for managing rSLE.
Numerous academic patient case studies have demonstrated the applicability, tolerance, and high effectiveness of CD19 CAR-T cell therapy in several autoimmune diseases, including SLE. It is anticipated that by targeting both CD19 and BCMA, GC012F might allow a more profound and broader elimination of disease-causing B-cells and plasma cells, potentially offering a more efficient and enduring therapeutic strategy for rSLE. Moreover, in the preclinical assessments, GC012F showed more effective elimination of antibody-secreting cells when compared to a CAR-T therapy that is solely targeted at CD19.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
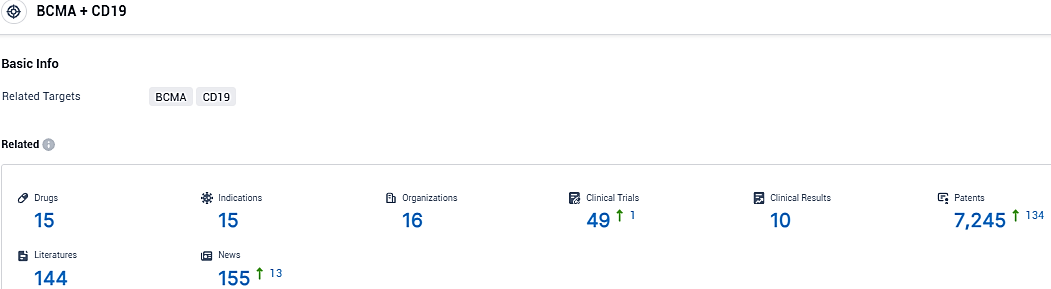
According to the data provided by the Synapse Database, As of November 30, 2023, there are 15 investigational drugs for the CD19/BCMA target, including 15 indications, 16 R&D institutions involved, with related clinical trials reaching 49, and as many as 7245 patents.
GC012F is Gracell’s FasTCAR-enabled BCMA/CD19 dual-targeting autologous CAR-T cell therapy, which aims to transform cancer and autoimmune disease treatment. GC012F is currently being evaluated in clinical studies in multiple hematological cancers as well as autoimmune diseases and has demonstrated a consistently strong efficacy and safety profile. An IIT has also been launched to evaluate GC012F for the treatment of rSLE and the IND application to study GC012F in rSLE has been cleared by the U.S. FDA.