Cartesian Therapeutics Administers First Dose in Phase 2 Study of Descartes-08 for Systemic Lupus Erythematosus
Cartesian Therapeutics, Inc., a biotechnology company at the clinical stage specializing in mRNA cell therapy for autoimmune diseases, revealed that the initial patient has been treated in its Phase 2 open-label clinical study assessing Descartes-08 in individuals with systemic lupus erythematosus.
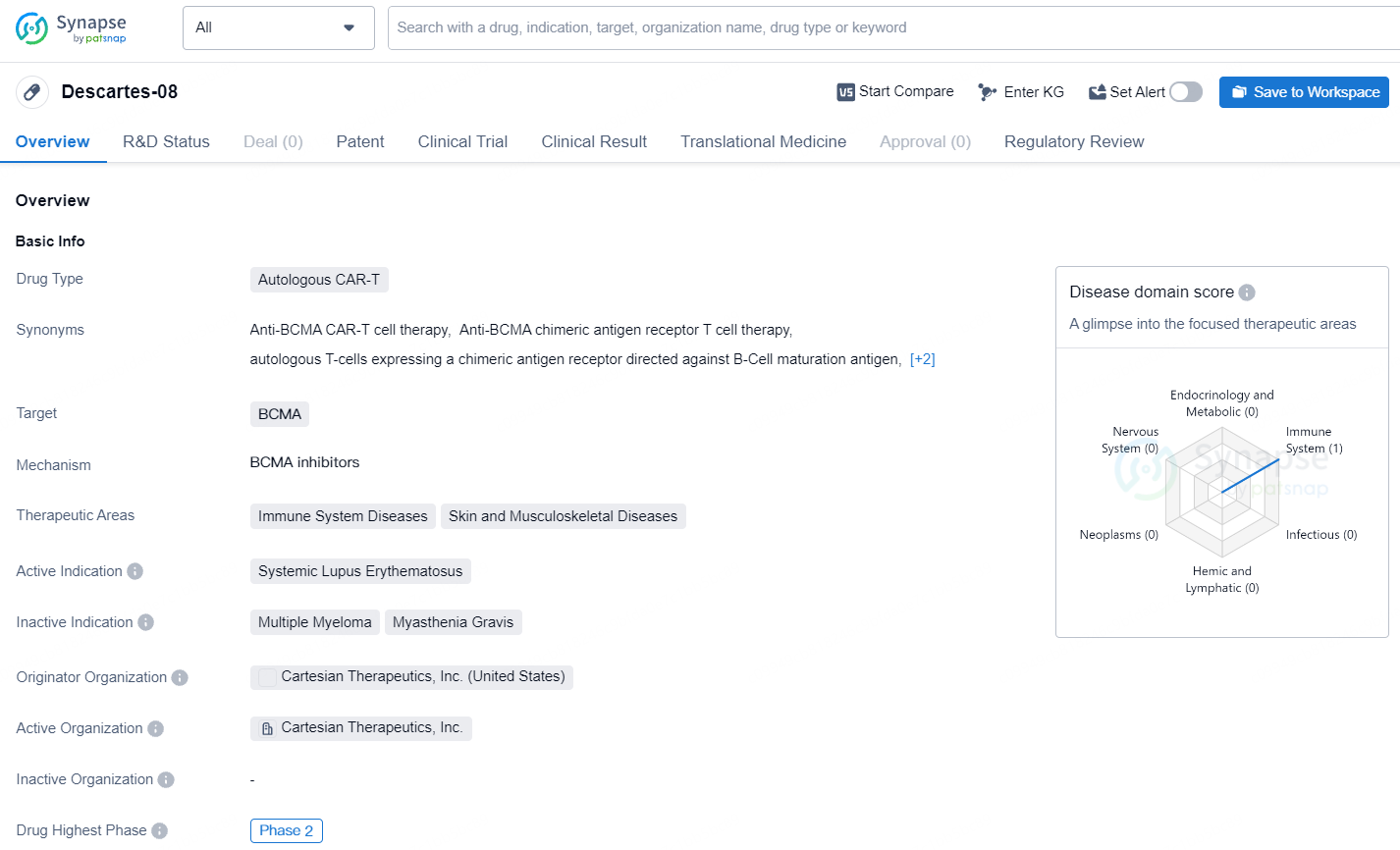
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Descartes-08, Cartesian's leading mRNA cell therapy candidate and a possible first-in-class mRNA-engineered chimeric antigen receptor T-cell therapy, is an autologous mRNA CAR-T product aimed at B-cell maturation antigen (BCMA). Unlike traditional DNA-based CAR-T cell therapies, mRNA CAR-T administration is designed to avoid the need for preconditioning chemotherapy and is not anticipated to have the risk of genomic integration that can lead to cancerous transformation.
Descartes-08 has already been administered to patients in a Phase 2 clinical trial for treating myasthenia gravis. So far, the safety profile from the MG trial supports its outpatient administration with minimal observation required.
“Administering the first patient with Cartesian’s autologous mRNA CAR-T cell therapy is a significant advancement for the lupus patient community,” stated Susan Manzi, M.D., M.P.H., Chair of the Medicine Institute at Allegheny Health Network and Medical Director for the Lupus Foundation of America. “Current treatment options for lupus patients are inadequate. I am optimistic that Descartes-08, when used as an outpatient therapy, will show early and durable clinical benefits for patients with certain B cell-mediated autoimmune diseases like lupus.”
The Phase 2 open-label trial (NCT06038474), which aims to enroll up to 30 adult patients, is designed to evaluate the safety and tolerability of outpatient administration of Descartes-08 without preconditioning chemotherapy in treating patients with moderate to severe SLE who are refractory to immunosuppressant therapy. Secondary outcome measures will evaluate overall disease activity.
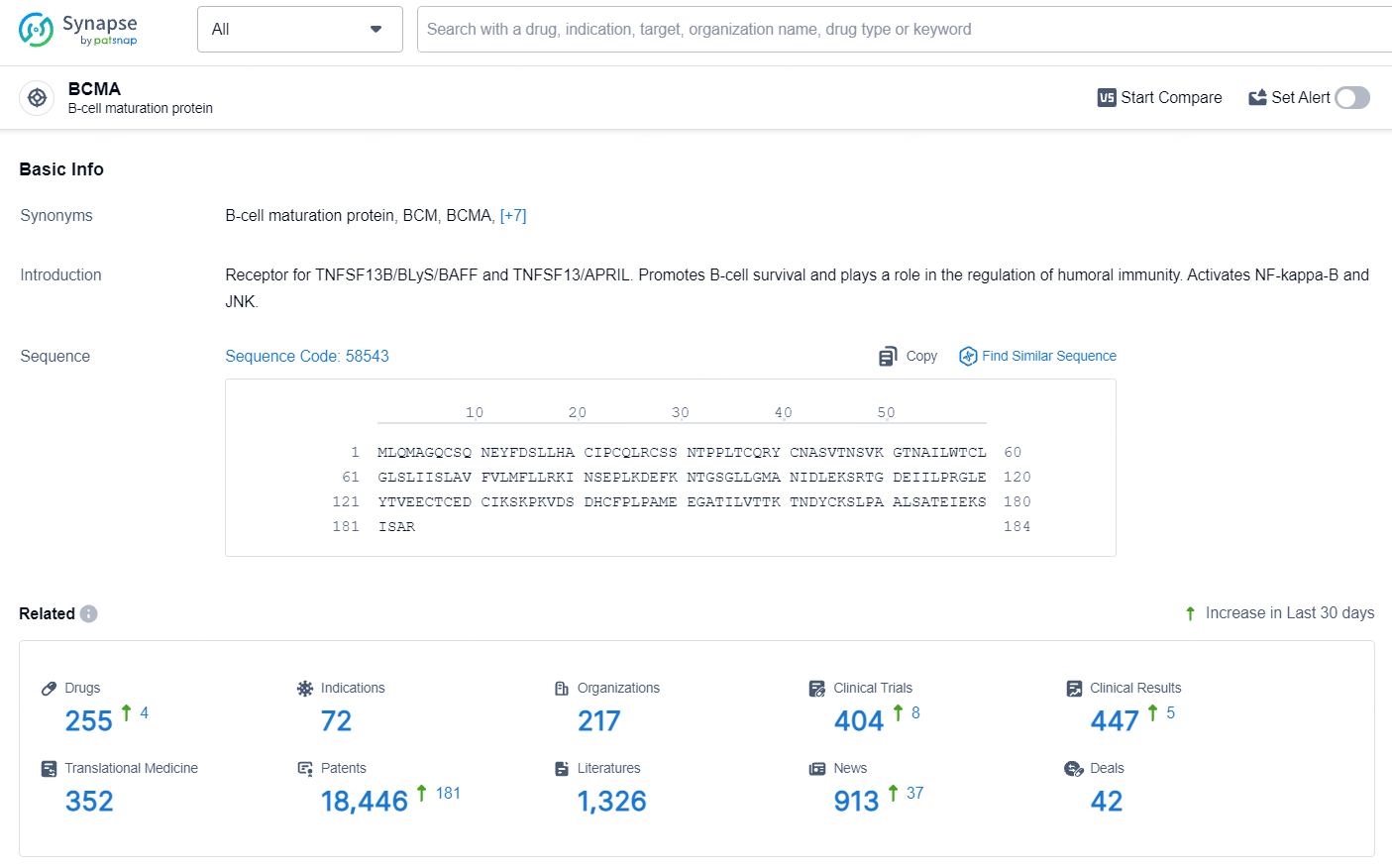
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of July 9, 2024, there are 255 investigational drugs for the BCMA target, including 72 indications, 217 R&D institutions involved, with related clinical trials reaching 404, and as many as 18446 patents.
Descartes-08 targets BCMA and is intended for the treatment of Systemic Lupus Erythematosus, a form of immune system disease.Descartes-08 has the potential to address significant unmet medical needs in the field of immune system diseases, particularly Systemic Lupus Erythematosus, and may offer new hope for patients with these conditions.