Unleashing the Power of Perindopril Erbumine: A Comprehensive Review on R&D Breakthroughs
Perindopril Erbumine's R&D Progress
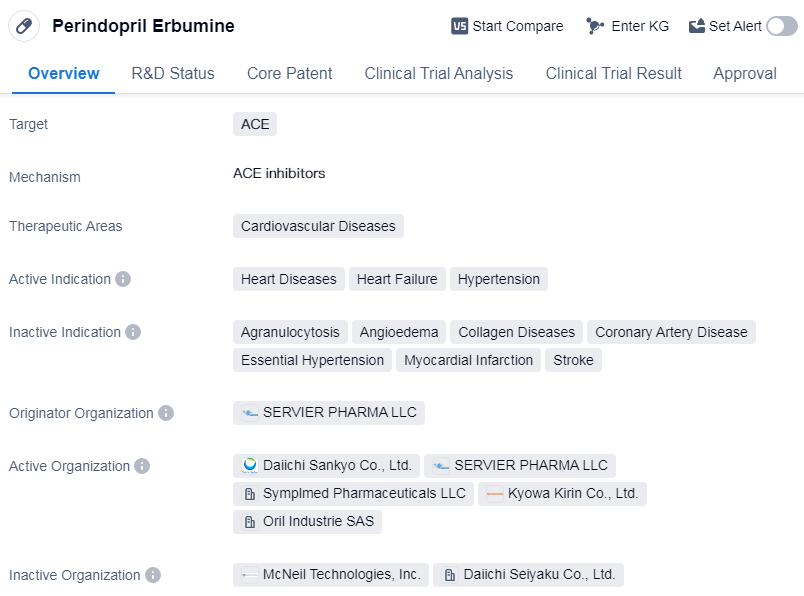
Perindopril Erbumine is a small molecule drug that falls under the therapeutic area of cardiovascular diseases. It specifically targets angiotensin-converting enzyme (ACE) and is primarily used for the treatment of heart diseases, heart failure, and hypertension.
The drug was first approved globally in January 1988, indicating that it has been in use for several decades. It is important to note that the information provided does not specify the specific indications for which Perindopril Erbumine has been approved. However, given its target of ACE and its therapeutic area of cardiovascular diseases, it can be inferred that the drug is likely used to manage conditions related to the cardiovascular system.
Perindopril Erbumine is developed by SERVIER PHARMA LLC, an originator organization in the pharmaceutical industry. As an approved drug, it has successfully completed the necessary clinical trials and regulatory processes to demonstrate its safety and efficacy for use in patients.
The fact that Perindopril Erbumine has reached the highest phase of approval globally indicates its widespread acceptance and recognition in the medical community. This suggests that the drug has undergone rigorous evaluation and has met the necessary standards for approval in multiple regions.
Given the drug's approval in the field of cardiovascular diseases, it is likely that Perindopril Erbumine has demonstrated positive outcomes in managing heart diseases, heart failure, and hypertension. However, without additional information, it is not possible to provide a comprehensive analysis of its efficacy, safety profile, or market potential.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Perindopril Erbumine: ACE inhibitors
ACE inhibitors, also known as angiotensin-converting enzyme inhibitors, are a class of drugs commonly used in biomedicine to treat various cardiovascular conditions such as hypertension (high blood pressure), heart failure, and certain kidney diseases.
These medications work by inhibiting the activity of the enzyme called angiotensin-converting enzyme (ACE), which plays a crucial role in the renin-angiotensin-aldosterone system (RAAS). The RAAS is responsible for regulating blood pressure and fluid balance in the body.
By blocking ACE, ACE inhibitors prevent the conversion of angiotensin I to angiotensin II. Angiotensin II is a potent vasoconstrictor, meaning it narrows the blood vessels and increases blood pressure. By reducing the production of angiotensin II, ACE inhibitors help dilate the blood vessels, lower blood pressure, and improve blood flow.
Furthermore, ACE inhibitors also decrease the secretion of aldosterone, a hormone that promotes salt and water retention, leading to a reduction in fluid volume and further lowering of blood pressure.
Overall, ACE inhibitors are valuable medications in the management of cardiovascular diseases, as they help relax blood vessels, decrease blood pressure, improve heart function, and reduce the workload on the heart. They are often prescribed in combination with other drugs to achieve optimal therapeutic effects.
Drug Target R&D Trends for Perindopril Erbumine
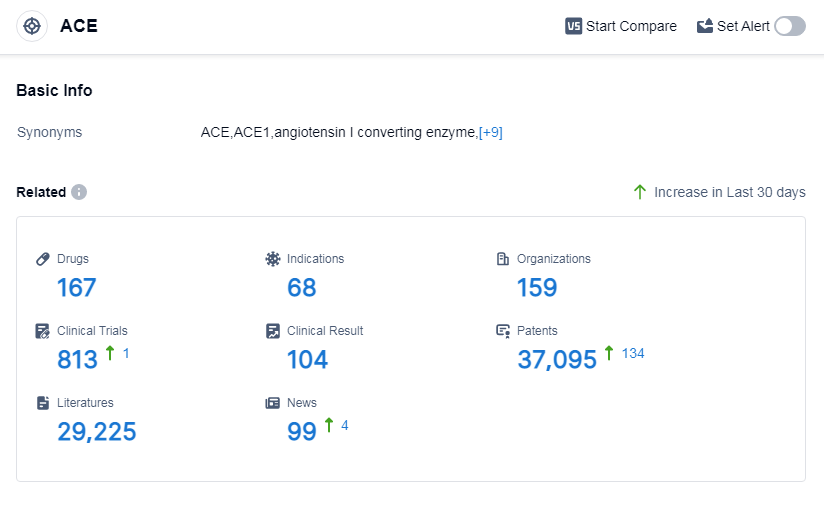
According to Patsnap Synapse, as of 14 Sep 2023, there are a total of 167 ACE drugs worldwide, from 159 organizations, covering 68 indications, and conducting 813 clinical trials.
The analysis of the target ACE reveals a competitive landscape with companies like Les Laboratoires Servier SAS, CHIESI Farmaceutici SpA, and Viatris Inc. leading the way in R&D progress. The most common indications for drugs under this target are hypertension, heart failure, and essential hypertension. Small molecule drugs dominate the market, but there is also potential in other drug types such as peptide drug conjugates and monoclonal antibodies. The European Union, China, the United States, and Japan are the key regions driving the development of ACE-related drugs. China, in particular, has shown significant progress. Overall, the target ACE presents opportunities for further research and development, with potential for innovative drugs and intense competition in the market.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Perindopril Erbumine is a small molecule drug developed by SERVIER PHARMA LLC. It targets ACE and is approved for use in the treatment of heart diseases, heart failure, and hypertension. With its approval in global markets, the drug has demonstrated its effectiveness and safety in managing cardiovascular conditions. However, further research and analysis are required to fully understand its potential impact in the pharmaceutical industry.