China NMPA Approves PADCEV™ for Advanced Urothelial Cancer Treatment
Astellas Pharma Inc. (TSE:4503, President and CEO: Naoki Okamura, “Astellas”) shared the news that China's National Medical Products Administration's Center for Drug Evaluation (CDE) has granted approval for PADCEV™ (enfortumab vedotin). This medication is indicated for adult individuals suffering from locally advanced or metastatic urothelial cancer (la/mUC) who have previously been treated with platinum-containing chemotherapy and inhibitors of programmed death receptor-1 (PD-1) or programmed death-ligand 1 (PD-L1).
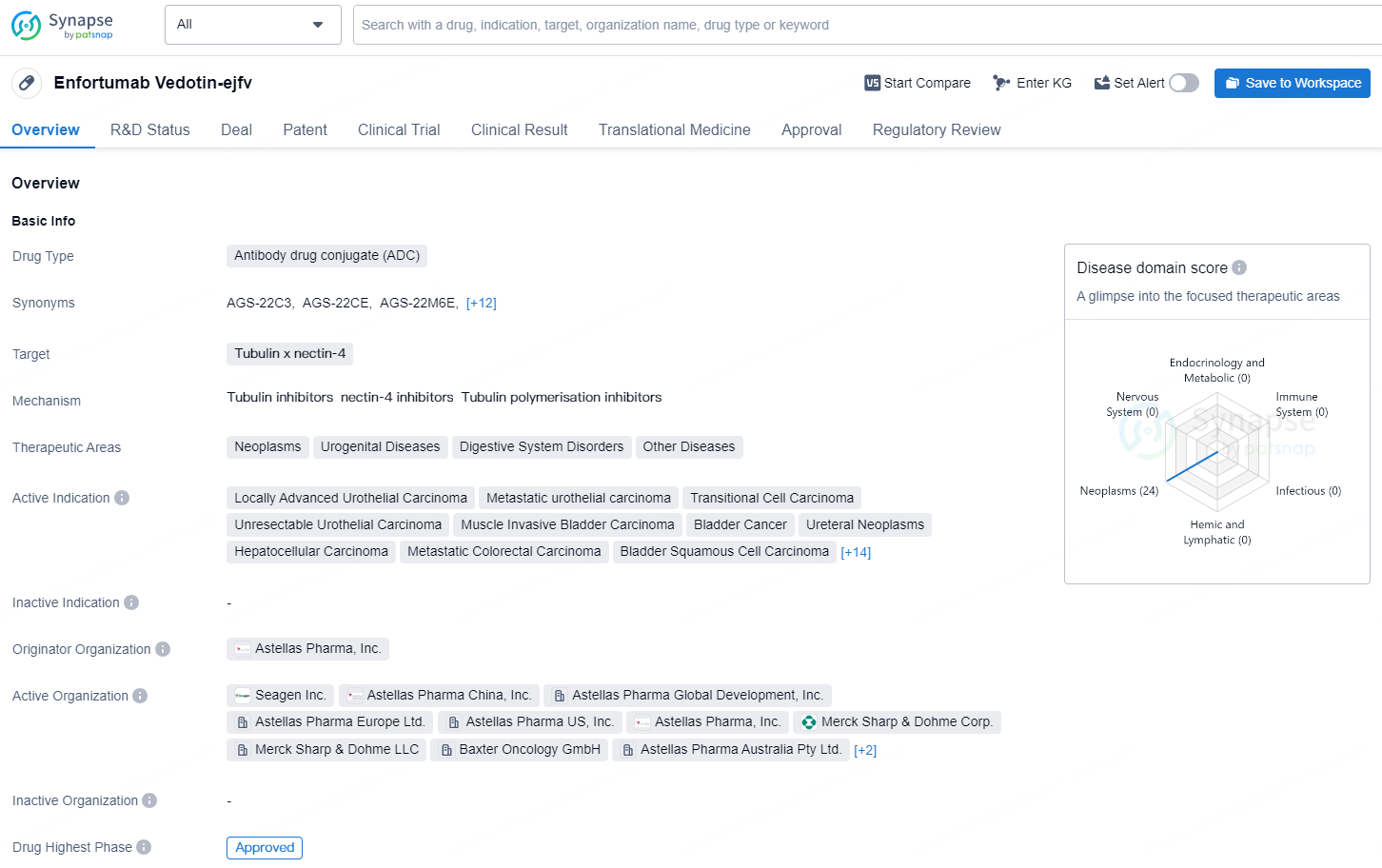
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
 Urothelial carcinoma is a challenging and frequently aggressive cancer impacting both the lower urinary tract (including the bladder and urethra) and the upper urinary tract (such as the ureter and renal pelvis).3,4,5 In 2022, over 92,000 people in China were diagnosed with bladder cancer, and there were about 41,000 deaths attributed to the illness.6 The prognosis is especially grim for individuals with locally advanced or metastatic urothelial carcinoma, emphasizing the critical need for novel treatments to prolong patient survival.
Urothelial carcinoma is a challenging and frequently aggressive cancer impacting both the lower urinary tract (including the bladder and urethra) and the upper urinary tract (such as the ureter and renal pelvis).3,4,5 In 2022, over 92,000 people in China were diagnosed with bladder cancer, and there were about 41,000 deaths attributed to the illness.6 The prognosis is especially grim for individuals with locally advanced or metastatic urothelial carcinoma, emphasizing the critical need for novel treatments to prolong patient survival.
Professor Guo Jun, Principal Investigator of the EV-203 trial and Director of the Department of Melanoma and Urological Oncology at Beijing Cancer Hospital, China, stated:
“On August 13, 2024, the NMPA formally approved enfortumab vedotin for treating adult patients with locally advanced or metastatic urothelial carcinoma (la/mUC) who have previously undergone platinum-based chemotherapy and PD-1/PD-L1 inhibitor therapy. This approval, supported by a global Phase 3 registration trial and a bridging study involving Chinese patients, is a significant development, giving patients in China access to this new antibody-drug conjugate (ADC) therapy.”
Professor Dingwei Ye, Academic Leader of the Department of Urology and Principal Expert in Urological Oncology MDT Management at Fudan University-Affiliated Cancer Hospital, China, remarked: “Enfortumab vedotin will be advantageous for patients in our country, offering a new therapeutic option for those with locally advanced or metastatic urothelial carcinoma (la/mUC) who have already received platinum-based chemotherapy and PD-1/PD-L1 inhibitors.”
Professor Zhisong He, Deputy Director of the Institute of Urology at Peking University First Hospital, China, commented: “Enfortumab vedotin, an ADC targeting Nectin-4, has received approval for the EV-203 indication, thereby broadening the range of treatment options available to doctors.”
Ahsan Arozullah, M.D., M.P.H., Senior Vice President and Head of Oncology Development at Astellas, added: “Our commitment remains towards advancing scientific innovations that bring about significant shifts in cancer treatment worldwide. The CDE's approval of enfortumab vedotin offers patients in China an additional therapeutic avenue for locally advanced or metastatic urothelial cancer, providing hope for better clinical outcomes for those affected.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
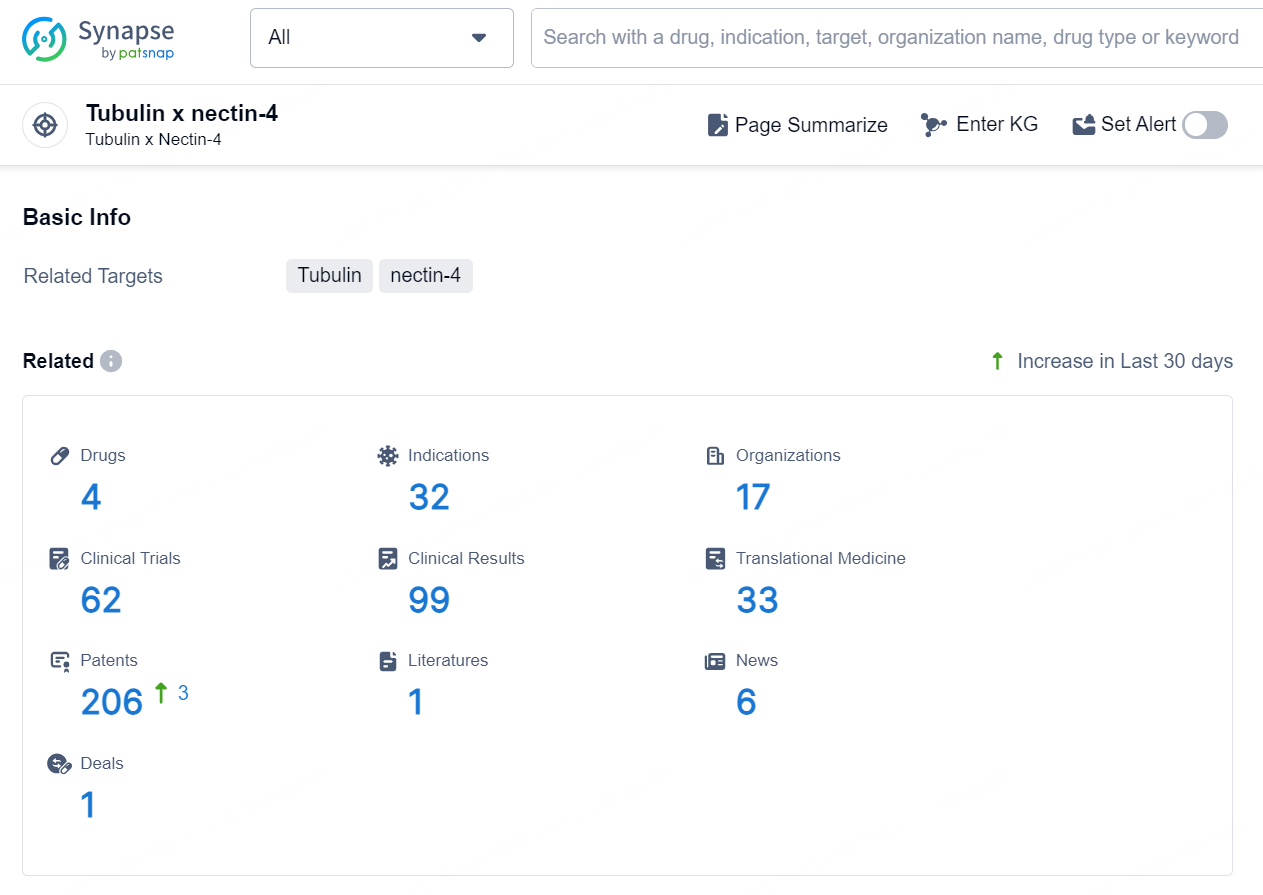
According to the data provided by the Synapse Database, As of August 22, 2024, there are 4 investigational drugs for the Tubulin x nectin-4 targets, including 32 indications, 17 R&D institutions involved, with related clinical trials reaching 62, and as many as 206 patents.
PADCEV (enfortumab vedotin) is a first-in-class antibody-drug conjugate (ADC) that is directed against Nectin-4, a protein located on the surface of cells and highly expressed in bladder cancer. Nonclinical data suggest the anticancer activity of enfortumab vedotin is due to its binding to Nectin-4-expressing cells, followed by the internalization and release of the anti-tumor agent monomethyl auristatin E (MMAE) into the cell, which result in the cell not reproducing (cell cycle arrest) and in programmed cell death (apoptosis).