China's Biopharma Breakthroughs: January 2025 Marks a New Era of Global Collaborations and Innovations
As the global pharmaceutical market continues to expand and China's biopharmaceutical industry rapidly advances, in January 2025, several companies announced collaborations with international pharmaceutical giants to launch a series of new drugs globally. From Innovent Biologics' collaboration with Roche on IBI3009 to Lepu Biopharma's licensing agreement with ArriVent for MRG007, these actions highlight the significant role Chinese biopharmaceuticals play in the globalization process.
1.Innovent Biologics joins forces with Roche to make IBI3009 a revolutionary force in treating small cell lung cancer
On January 2, 2025, Innovent Biologics announced it had granted Roche the global exclusive rights to the new drug IBI3009. According to the terms of the agreement, Innovent Biologics has transferred to Roche the exclusive global rights to develop, manufacture, and commercialize IBI3009. The parties plan to jointly advance the early developmental stages of this antibody-drug conjugate (ADC), with Roche leading the subsequent clinical development. As part of the deal, Innovent Biologics will receive an upfront payment of $80 million, with the potential to earn up to $1 billion in milestone payments and royalties based on sales.
IBI3009 is a next-generation ADC developed by Innovent Biologics, targeting Delta-like ligand 3 (DLL3), a marker that is lowly expressed in normal tissues but highly expressed in specific cancer types such as small cell lung cancer (SCLC). By specifically binding to DLL3, IBI3009 can precisely target cancer cells without harming healthy cells, providing new hope for treatment in advanced SCLC patients. This drug utilizes Innovent Biologics' proprietary new class topoisomerase I inhibitor platform for efficient payload delivery and intracellular toxin release in tumor cells, showing promising antitumor activity and safety.
2.WuXi Biologics partners with Candid Therapeutics to advance a novel trispecific antibody therapy using the WuXiBody™ platform
On January 7, 2025, WuXi Biologics entered into a landmark research services collaboration agreement with Candid Therapeutics, marking a new phase in their partnership in the innovation of therapeutic development. Under the agreement, Candid will obtain global rights to a trispecific antibody developed on WuXi Biologics' WuXiBody™ platform, currently in preclinical development, designed to target three different antigen sites: BCMA, CD20, and CD19. BCMA is highly expressed in hematologic malignancies such as multiple myeloma, while CD20 and CD19 are primarily found on the surface of B cells, playing crucial roles in various B-cell-related cancers including non-Hodgkin's lymphoma. This antibody can not only recognize and bind to specific antigens on cancer cells but also activate the patient's immune system, particularly T cells, thereby more effectively clearing tumor cells.
WuXi Biologics' WuXiBody™ platform offers unique advantages for this trispecific antibody, enabling simultaneous targeting of tumor cell antigens CD20 and CD19 as well as the T-cell surface activation molecule BCMA, promoting close contact between T cells and tumor cells, enhancing T-cell-mediated cytotoxicity. Furthermore, through this triple targeting mechanism, the antibody may also inhibit the ability of tumor cells to evade immune surveillance, further enhancing therapeutic efficacy. The financial terms of this collaboration are also notable, with WuXi Biologics receiving an undisclosed upfront payment, eligible for subsequent development and sales milestone payments totaling up to $925 million, plus royalties on post-commercialization sales.
3.Duality Biologics Reaches Dual-Specificity ADC Collaboration Agreement with Avenzo Therapeutics
On January 8, 2025, Duality Biologics announced an exclusive licensing agreement with Avenzo Therapeutics, granting Avenzo the global rights (excluding Greater China) to develop, manufacture, and commercialize the dual-specificity antibody-drug conjugate (ADC) EGFR/HER3, DB-1418/AVZO-1418. According to the agreement terms, Duality Biologics will receive an upfront payment of $50 million and is eligible for up to $1.15 billion in developmental, regulatory, and commercial milestones, in addition to royalties on sales in Avenzo's designated regions.
DB-1418/AVZO-1418 is an innovative dual-specificity ADC that targets EGFR and HER3, which are overexpressed antigens in various cancer types. By targeting both receptors, DB-1418 enhances selective recognition of cancer cells and promotes more effective internalization, delivering its cytotoxic payload – a topoisomerase I inhibitor – directly into the tumor cells to disrupt their DNA replication process, leading to cancer cell death. The ADC leverages Duality Biologics’ proprietary DIBAC platform, ensuring both efficient targeting and stable cargo-carrying capabilities of the antibody, overcoming resistance issues common with traditional single-target therapies.
4.Sciwind Biosciences and Verdiva Bio Seal Major Agreement to Propel Innovation in Metabolic Disease Therapeutics
On January 10, 2025, Sciwind Biosciences announced a global development and commercialization license and partnership agreement with Verdiva Bio Limited, focusing on innovative therapies in the cardiovascular metabolic disorder sector. A key product under this collaboration is Ecnoglutide Oral, a potentially first-in-class medication in Phase II clinical trial preparation that requires only once-weekly dosing. As a GLP-1 receptor agonist, Ecnoglutide mimics the action of endogenous GLP-1 by activating the glucagon-like peptide-1 receptor (GLP-1R) which is prevalent in multiple organs including the pancreas, gastrointestinal tract, and heart. Activation of GLP-1R promotes insulin secretion, inhibits glucagon release, and slows gastric emptying, thereby aiding in blood sugar control and weight reduction. Moreover, Ecnoglutide possesses cAMP bias, which may offer unique advantages in activating downstream signaling pathways.
Additionally, the partnership agreement includes two amylin receptor agonists, one in oral form and the other as a subcutaneous injection. Amylin, secreted by β-cells alongside insulin, assists in postprandial glucose regulation by delaying gastric emptying, reducing food intake, and lowering postprandial glucose peaks. These amylin receptor agonists are designed to complement the effects of GLP-1 receptor agonists, particularly in terms of weight reduction and improved glucose control. By acting directly on the gastrointestinal tract and through central nervous system mechanisms regulating appetite, co-utilizing amylin with GLP-1 offers a potentially more effective and comprehensive metabolic disease management strategy.
This partnership is valued at up to $2.47 billion, including nearly $70 million upfront and up to $2.4 billion in potential development, regulatory, and commercialization milestone payments. Further, Sciwind Biosciences will receive royalties on post-commercialization sales. This substantial financial support not only underscores Verdiva Bio's confidence in the market potential of these candidate drugs but also provides robust financial backing to Sciwind Biosciences to expedite the progress of other projects in its development pipeline.
5. Keymed Biomedical’s CD38 Monoclonal Antibody CM313
On January 10, 2025, Keymed Biomedical announced an exclusive licensing agreement with Timberlyne Therapeutics for the global rights (excluding Mainland China, Hong Kong, Macau, and Taiwan) to CM313, a humanized monoclonal antibody targeting CD38 developed in-house. CM313 is an innovative therapeutic agent that specifically binds to and eliminates tumor cells expressing the CD38 glycoprotein, particularly long-lived plasma cells (LLPCs), thereby preventing antibody-mediated platelet destruction and restoring normal platelet production and function. Additionally, CM313 has the ability to modulate immune cell activity, making it a promising candidate for the treatment of relapsed/refractory multiple myeloma, lymphoma, and other autoimmune diseases. Its mechanism of action extends beyond direct induction of tumor cell apoptosis, also involving indirect tumor cell killing via antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and antibody-dependent cellular phagocytosis (ADCP).
Under the terms of the agreement, Keymed Biomedical will receive an upfront and near-term payment of $30 million and will become the largest shareholder of Timberlyne, holding a 25.79% equity stake. Furthermore, upon achieving certain sales and development milestones, Keymed is eligible to receive up to an additional $337.5 million, as well as tiered royalties based on net sales. Notably, Timberlyne has successfully completed a $180 million Series A financing round, attracting investments from prominent institutions such as Bain Capital Life Sciences and Venrock Healthcare Capital Partners.
6. Kelun Botai and Harbour BioMed Partner for Global Expansion of TSLP Monoclonal Antibody SKB378/HBM9378
On January 10, 2025, Kelun Botai and Harbour BioMed jointly announced an exclusive licensing agreement with Windward Bio AG for SKB378/HBM9378, a monoclonal antibody targeting thymic stromal lymphopoietin (TSLP), which they co-developed. SKB378/HBM9378 is a fully human monoclonal antibody designed to inhibit TSLP-mediated signaling by blocking the interaction between TSLP and its receptor, thereby reducing inflammatory responses. The drug has already completed a Phase I clinical trial in China for moderate-to-severe asthma, demonstrating good safety and preliminary efficacy.
Under the terms of the agreement, Kelun Botai and Harbour BioMed will receive up to $970 million in upfront and milestone payments from Windward Bio, along with tiered royalties ranging from single-digit to double-digit percentages based on net sales. The upfront and near-term payments total $45 million, consisting of both cash consideration and equity in Windward Bio’s parent company. This collaboration follows a NewCo model, meaning that Windward Bio will assume full responsibility for the global (excluding Greater China and select Southeast and West Asian countries) research, development, manufacturing, and commercialization of SKB378/HBM9378. With further clinical development plans underway, particularly in chronic obstructive pulmonary disease (COPD), SKB378/HBM9378 has the potential to become a novel treatment option for this serious respiratory disease.
7. Simcere Zaiming and AbbVie Join Forces to Advance a New Breakthrough in Multiple Myeloma Treatment
On January 13, 2025, Simcere Zaiming entered into a significant development and option licensing agreement with AbbVie, a global pharmaceutical leader. This collaboration focuses on SIM0500, a GPRC5D/BCMA/CD3 trispecific antibody independently developed by Simcere Zaiming. Currently in Phase I clinical trials in both China and the United States, SIM0500 has also been granted Fast Track designation by the U.S. Food and Drug Administration (FDA). As a humanized trispecific antibody targeting multiple myeloma, SIM0500 simultaneously binds to GPRC5D, BCMA, and CD3 on T cells. This multi-target approach enhances tumor cell selectivity and cytotoxicity while improving the safety and efficacy of treatment.
Under the terms of the agreement, Simcere Zaiming will receive an upfront payment from AbbVie and is eligible for up to $1.055 billion in option and milestone payments. Additionally, Simcere Zaiming will receive tiered royalties based on net sales of SIM0500 outside Greater China, while AbbVie will be entitled to tiered royalties on net sales within the Greater China region. This agreement not only provides Simcere Zaiming with direct financial benefits but also enables the company to share in the global commercial success of SIM0500.
8. Bispecific Antibody ICP-B02 (CM355): InnoCare Pharma and Keymed Biomedical Collaborate to Pioneer a New Frontier in Tumor Immunotherapy
On January 20, 2025, InnoCare Pharma and Keymed Biomedical jointly announced a significant licensing agreement with Prolium Bioscience, granting Prolium the rights to develop and commercialize ICP-B02 (CM355), a bispecific antibody. ICP-B02 is an innovative drug designed to simultaneously recognize and bind to CD20-positive tumor cells and CD3-positive T cells. This dual-targeting mechanism enables ICP-B02 to guide the immune system’s T cells toward tumor cells and activate them to effectively eliminate cancer cells through T cell-dependent cytotoxicity (TDCC). Currently, ICP-B02 is undergoing Phase I/II clinical trials in China, primarily targeting relapsed or refractory non-Hodgkin lymphoma (NHL), including follicular lymphoma (FL) and diffuse large B-cell lymphoma (DLBCL).
Under the terms of the agreement, the two companies will receive payments totaling up to $520 million, including upfront and near-term payments, as well as additional payments upon achieving specified clinical development, regulatory, and commercialization milestones. Additionally, they will retain a minority equity stake in Prolium Bioscience and are entitled to tiered royalties based on future net sales of the product. Prolium has obtained the rights to develop, register, manufacture, and commercialize ICP-B02 in non-oncology indications worldwide and in oncology indications outside Asia.
9. Lepu Biopharma and ArriVent Collaborate to Advance the Globalization of the MRG007 Antibody-Drug Conjugate
On January 22, 2025, Lepu Biopharma entered into an exclusive global licensing agreement with ArriVent BioPharma, granting ArriVent the rights to develop, manufacture, and commercialize the innovative antibody-drug conjugate (ADC) MRG007 outside the Greater China region. MRG007 is designed for the treatment of gastrointestinal cancers. Under the agreement, ArriVent gains exclusive rights to MRG007 in all territories outside Mainland China, Hong Kong, Macau, and Taiwan. This collaboration represents a significant milestone for Lepu Biopharma, not only facilitating the advancement of MRG007’s clinical development but also marking an important step in the company’s global expansion strategy.
According to the agreement, Lepu Biopharma will receive a total of $47 million in upfront and near-term milestone payments, with additional development, regulatory, and sales milestone payments of up to $1.16 billion. Furthermore, Lepu Biopharma is entitled to tiered royalties based on net sales of MRG007 outside Greater China. This financial arrangement ensures short-term capital inflow for the company while laying a solid foundation for its future success. Moreover, it reflects the international market’s recognition and confidence in MRG007’s potential, signaling that this drug could become a key treatment option for gastrointestinal cancers.
Summary
January 2025 witnessed a series of significant events in the international expansion of Chinese innovative drugs. These strategic collaborations not only provided substantial financial returns and growth opportunities for Chinese pharmaceutical companies but also expanded global treatment options for patients. For instance, Innovent Biologics accelerated the development of its novel small-cell lung cancer ADC, IBI3009, through a partnership with Roche. Similarly, DualityBio licensed the global rights of its EGFR/HER3 bispecific ADC, DB-1418, to Avenzo Therapeutics, further strengthening its presence in the oncology sector. Additionally, Sciwind Biosciences’ collaboration with Verdiva Bio focused on metabolic diseases, demonstrating the innovative capabilities of Chinese companies beyond oncology.
Notably, the financial terms behind these agreements are equally impressive. For example, Keymed Biomedical secured up to $337.5 million in additional payments, while Lepu Biopharma stands to receive up to $1.16 billion in milestone payments. These transactions not only highlight the global market’s high regard for the potential of Chinese innovative drugs but also underscore the strong demand from multinational corporations for high-quality, high-potential pharmaceutical assets when seeking collaboration opportunities.
How to get the latest progress on drug deals?
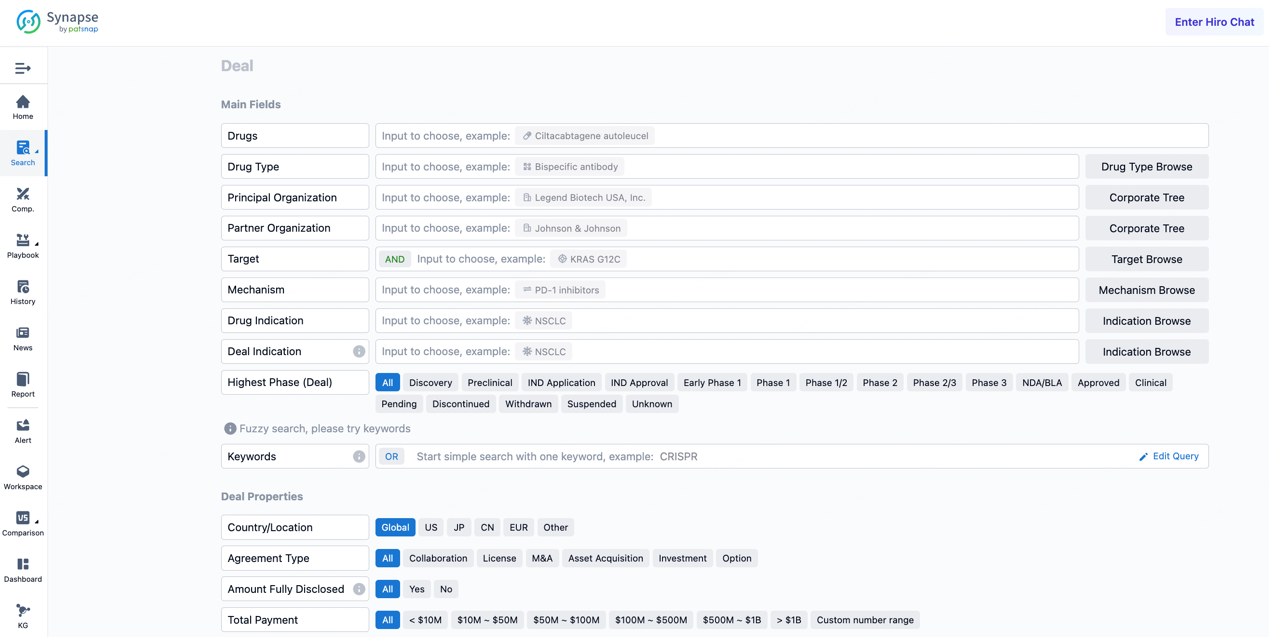
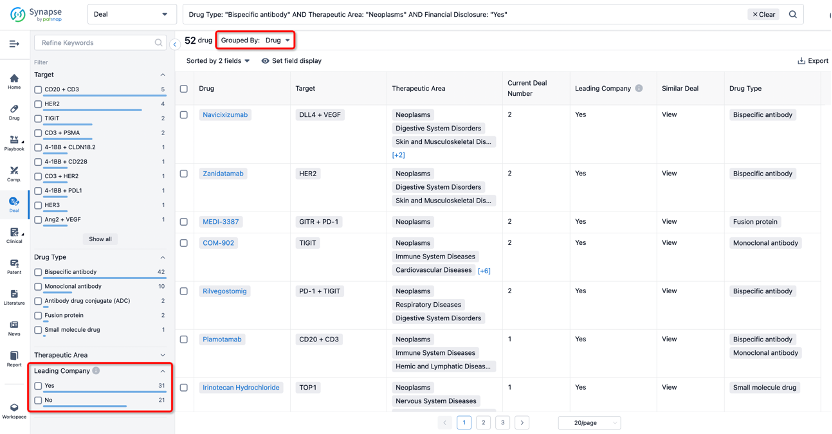
If you would like to access the latest transaction event information, you can click on the 'Deal' module from the homepage of the Synapse database. Within the Deal module, you can search for global pharmaceutical transaction information using labels such as Drugs, Organization, Target, Drug Type, Deal Date.
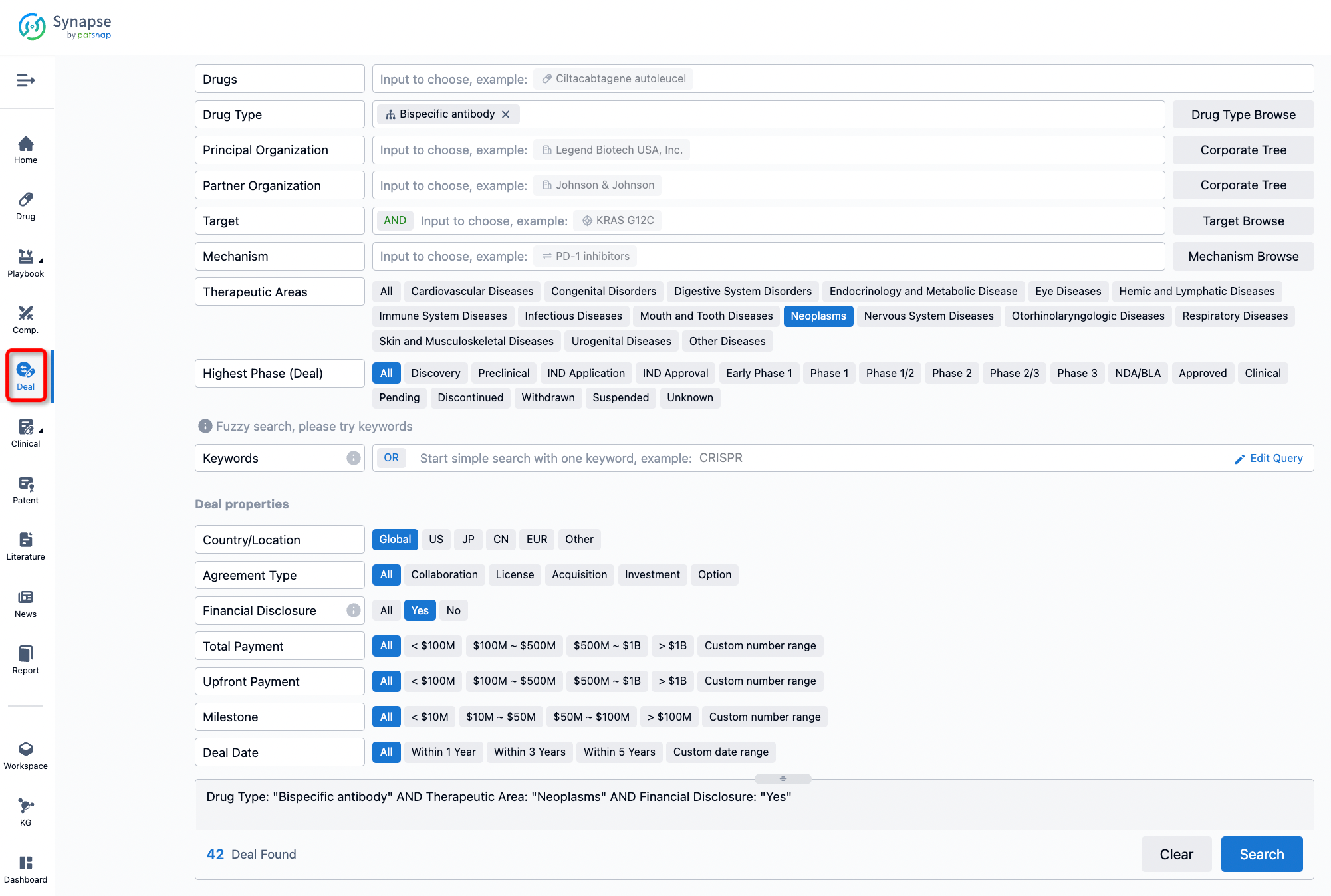
Furthermore, you can obtain the original link to the transaction coverage by clicking on the "Deal Name."
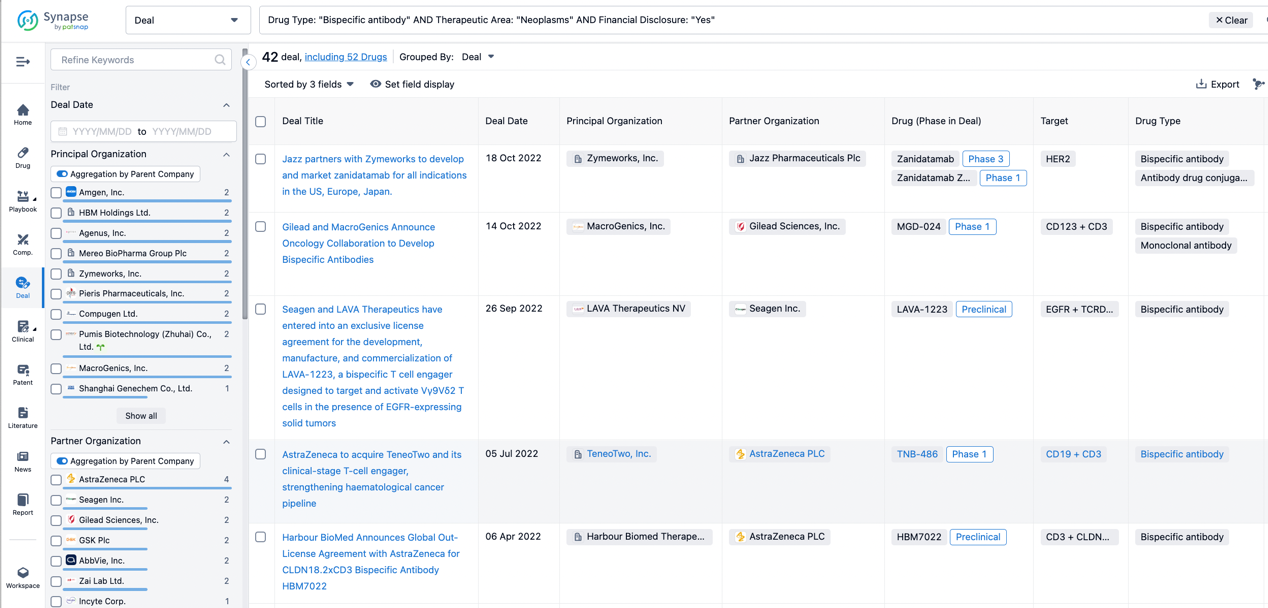
In the analysis view, you can see the most active assignors, assignees, popular targets, and other dimensions of analysis, as well as the distribution of research and development statuses at the time of the transaction, to help you better understand the search results.
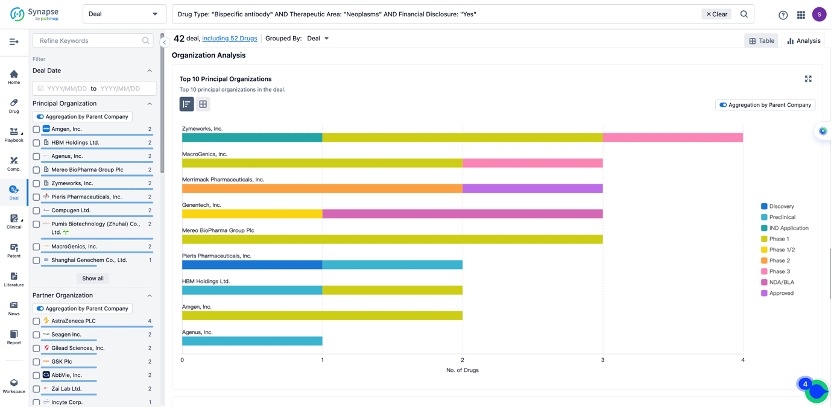
The Synapse database also supports the ability to view current transactions from the dimension of "drugs" (by selecting "drugs" from the "Adjust Dimension" dropdown menu above). Targeting transactions involving renowned pharmaceutical companies that are of interest to the industry, such as Merck, Roche, etc., Synapse has identified a group of "leading companies" through drugs that have achieved global sales exceeding 1 billion US dollars in 2022. Transactions involving drugs from these leading companies can be filtered by clicking on the "Leading Company" tag on the left-hand side.
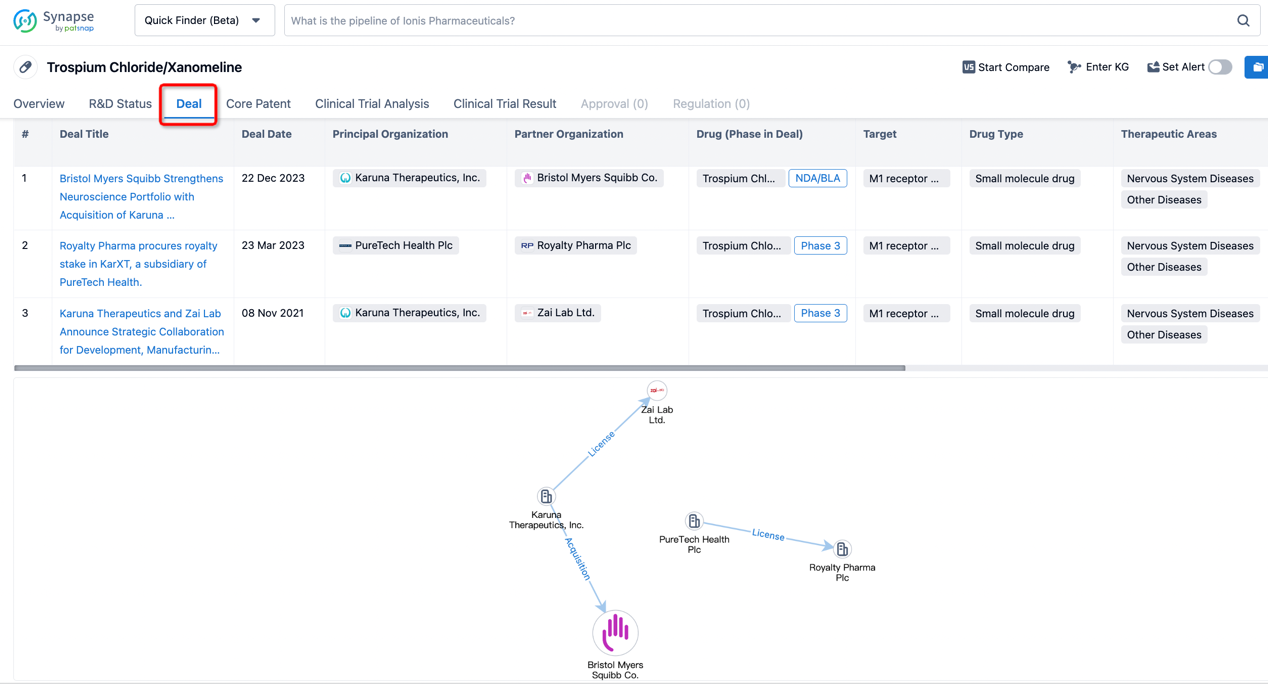
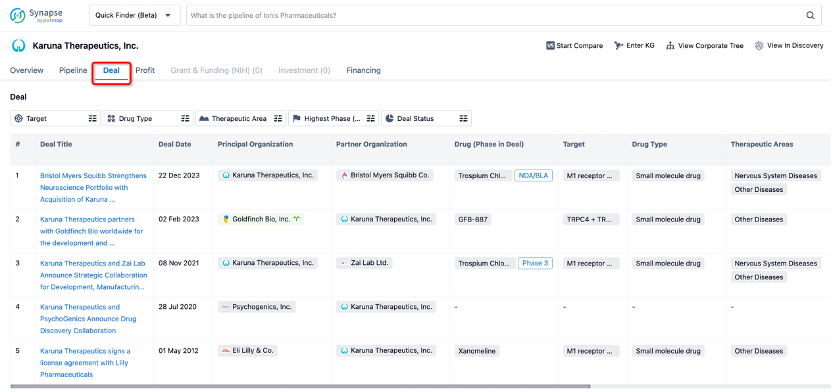
In addition to the drug transaction module, you can also view related transaction history on the drug detail page and the institution detail page.
Click on the image below to explore new pharmaceutical funding transactions!