CHMP has adopted a negative opinion on lecanemab for the EU
The European Medicines Agency's Committee for Medicinal Products for Human Use has announced it has given a negative opinion on the Marketing Authorization Request for lecanemab by BioArctic AB’s partner Eisai for Alzheimer's treatment.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
BioArctic is in discussions with Eisai about future steps. Lecanemab has received approval in the U.S., Japan, China, South Korea, Hong Kong, and Israel and is being sold in the U.S., Japan, and China.
Eisai oversees lecanemab's clinical development, market approval applications, and commercialization for Alzheimer's disease. BioArctic has rights to market lecanemab in the Nordic region if it gets European approval. Eisai and BioArctic are preparing for joint marketing efforts there.
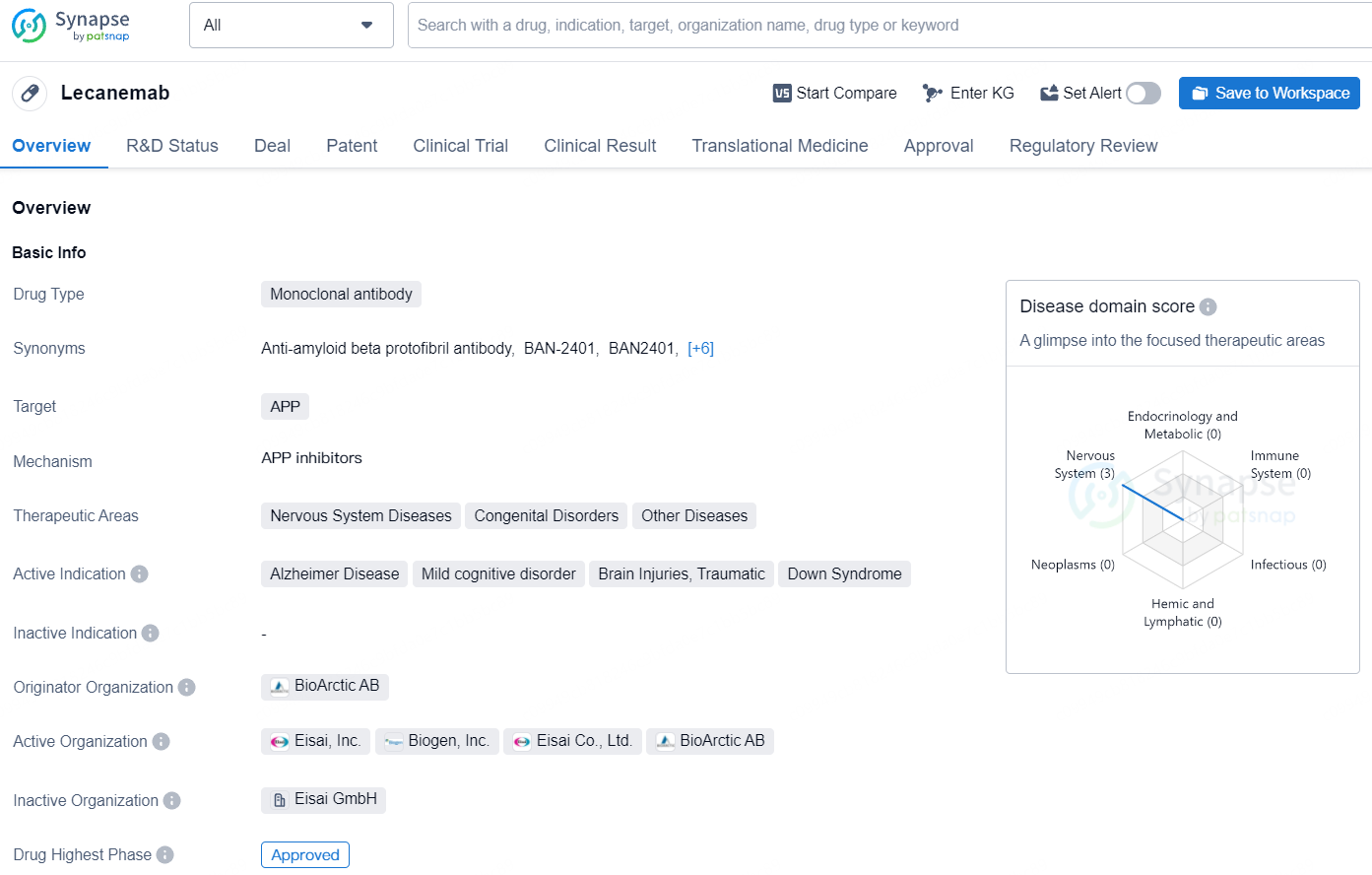
Lecanemab is a product of BioArctic and Eisai's research collaboration. It's a humanized IgG1 monoclonal antibody targeting aggregated amyloid-beta. Approved in the U.S., Japan, China, South Korea, Hong Kong, and Israel, lecanemab treats early Alzheimer's disease.
Approvals for lecanemab were based on the Phase 3 Clarity AD study, where it met all key endpoints with significant results. Results were shared at the 2022 Clinical Trials on Alzheimer's Disease conference and published in the New England Journal of Medicine.
Eisai has sought lecanemab approval in 11 other regions, including the EU. A supplemental Biologics License Application for intravenous maintenance dosing was filed with the U.S. FDA in March 2024. In May 2024, a rolling submission for maintenance dosing via subcutaneous injections, designed for patient convenience, began under Fast Track status in the U.S.
Since July 2020, Eisai has been conducting a Phase 3 study on individuals with preclinical AD. AHEAD 3-45 is a U.S. public-private partnership including the Alzheimer's Clinical Trial Consortium, funded by the National Institute on Aging and Eisai.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
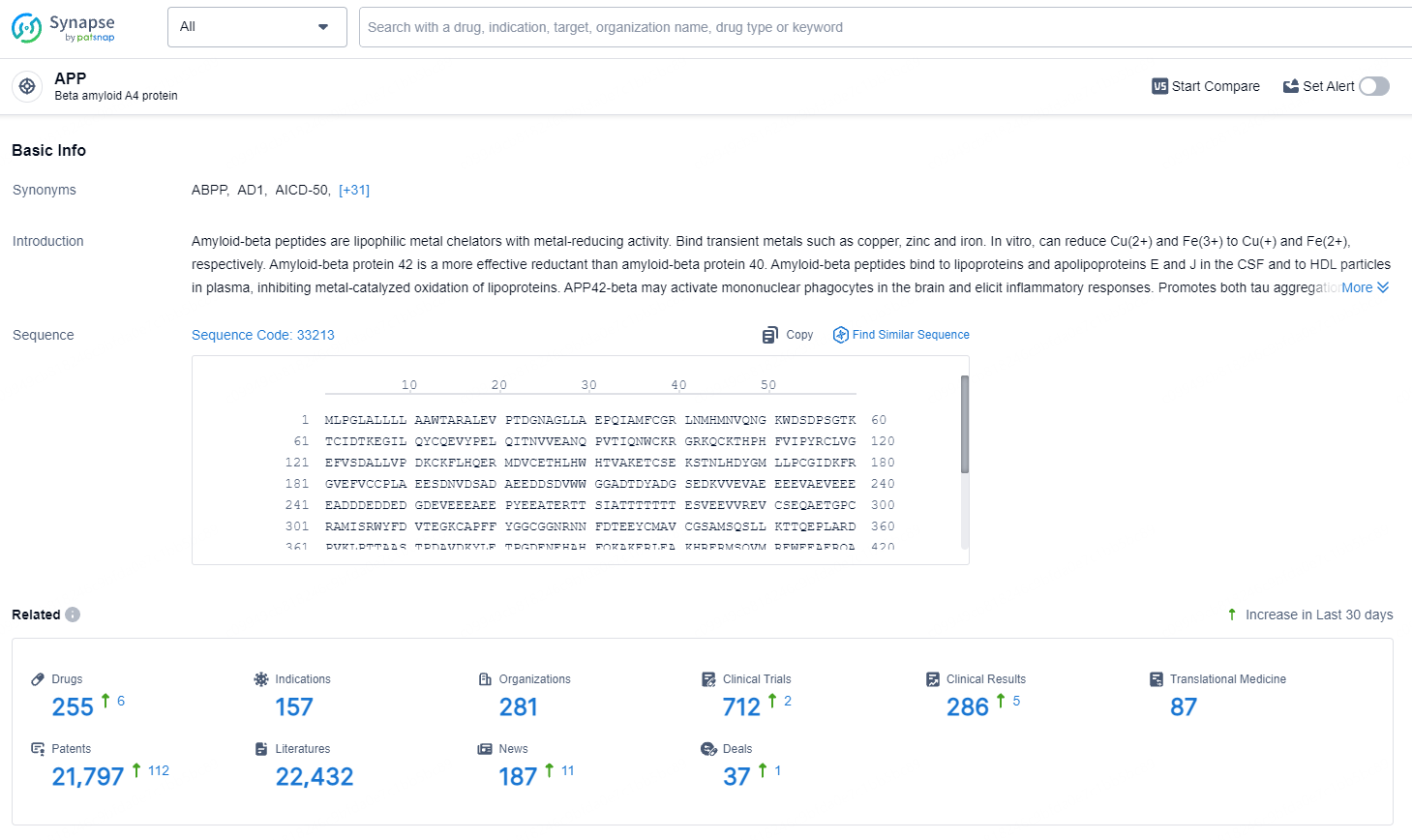
According to the data provided by the Synapse Database, As of August 1, 2024, there are 255 investigational drugs for the APP target, including 157 indications, 281 R&D institutions involved, with related clinical trials reaching 712, and as many as 21797 patents.
Lecanemab is a monoclonal antibody drug developed by BioArctic AB, targeting the amyloid precursor protein (APP). The approval of Lecanemab represents a major milestone in the pharmaceutical industry, particularly in the field of biomedicine. Its approval offers hope for patients suffering from Alzheimer's disease, Mild cognitive disorder, Brain Injuries, and Down Syndrome, as well as for those affected by Nervous System Diseases, Congenital Disorders, and Other Diseases. The regulatory designations given to Lecanemab also highlight its potential to offer innovative and effective treatment options, and its approval will likely have a significant impact on the pharmaceutical landscape and patient care.